Comprehensive Research & Discovery Support
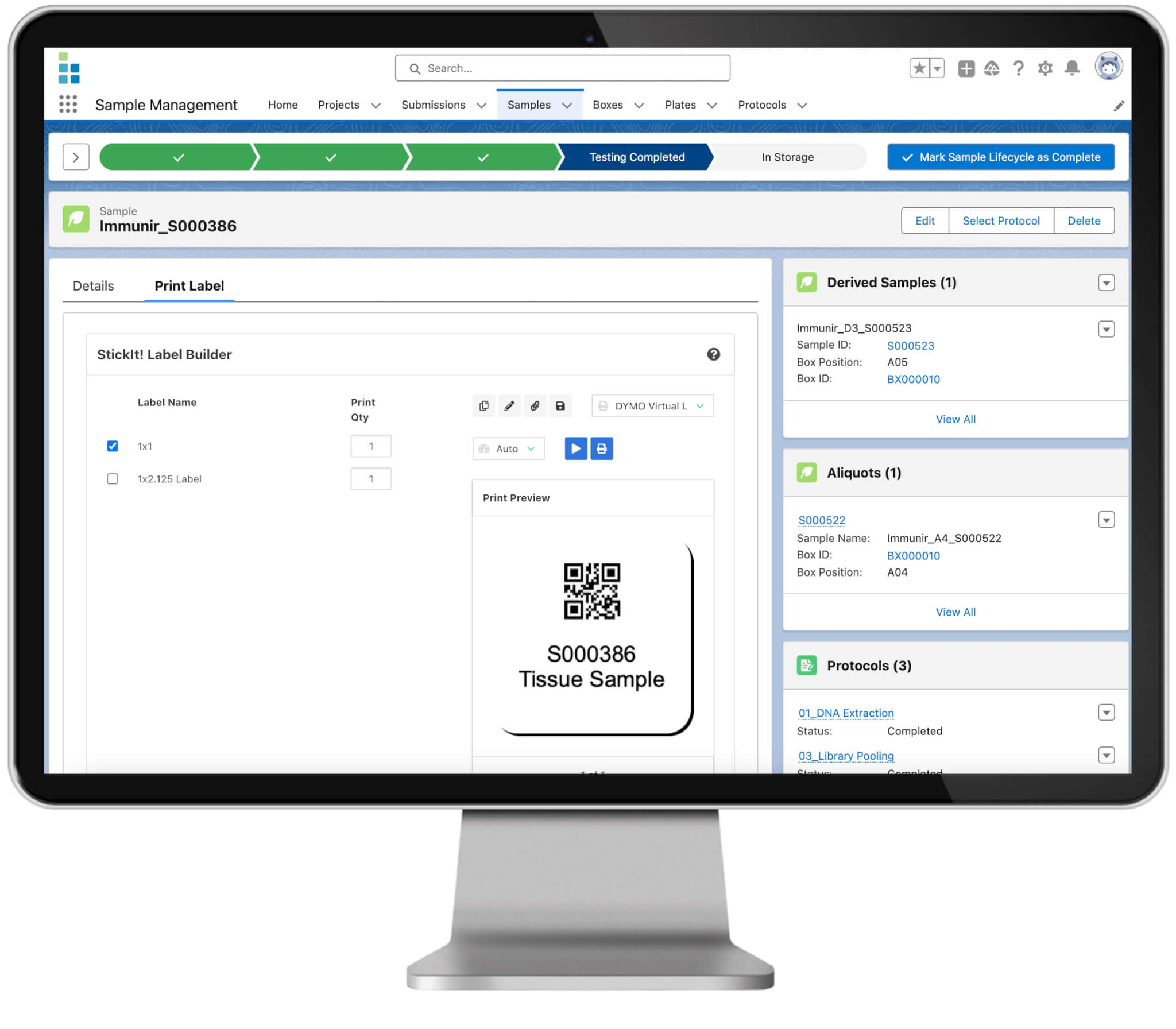
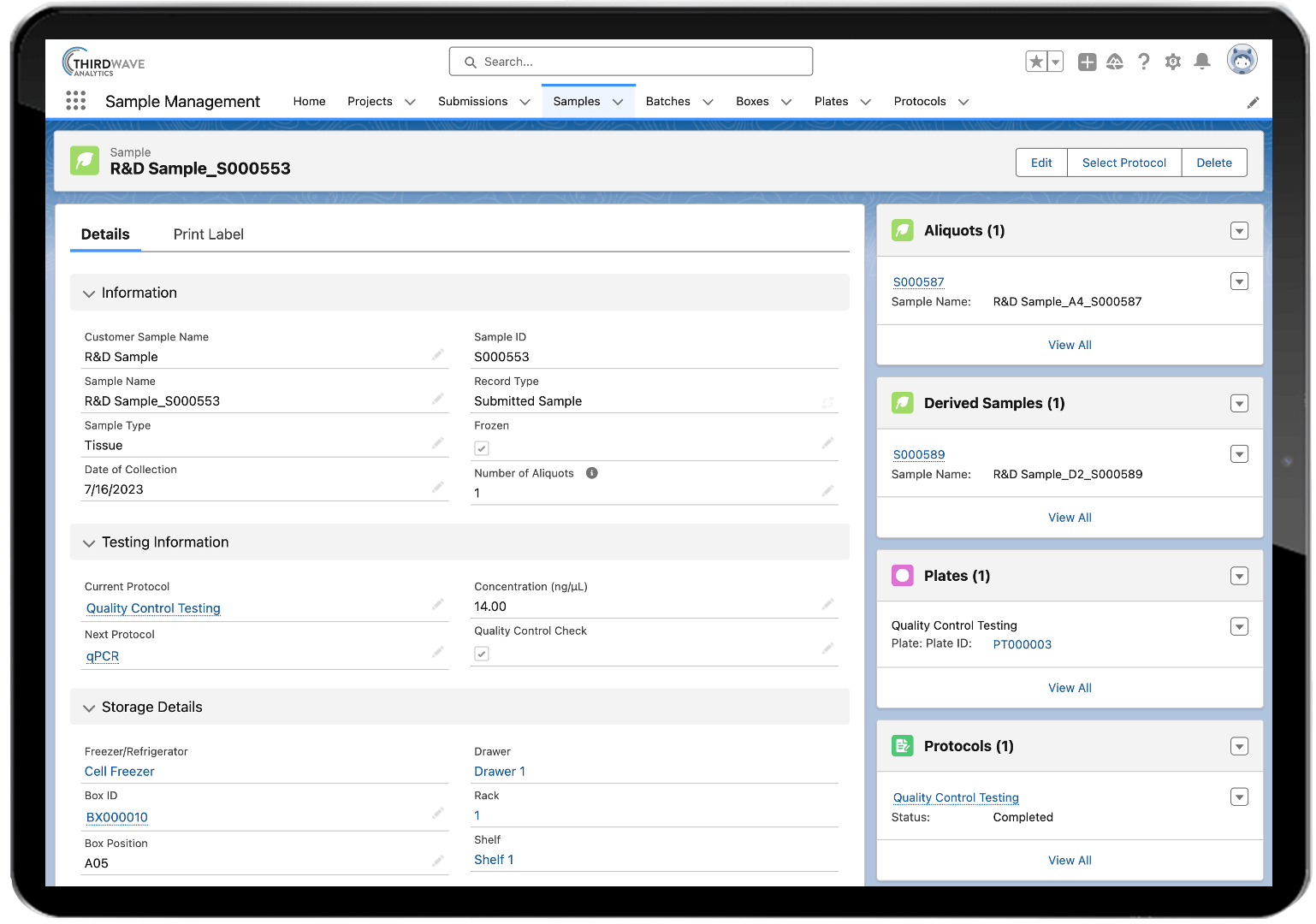
Why use a LIMS in pharma? With it’s comprehensive, all-inclusive functionality, Lockbox LIMS supports every phase of the discovery process, from basic research, to clinical trials and pharmaceutical GMP quality control. LIMS software is one of the most important tools in pharmaceutical research and drug development. Lockbox LIMS organizes every aspect of biotech or pharmaceutical laboratories activities so researchers can focus on breakthroughs, not on data integrity.