Streamline Lab Document Control with Lockbox QMS
Summary
The Lockbox QMS Document Control module fulfills your laboratory’s need for a cloud-based document management system. This module allows users to:
- Create a single, centralized repository for all quality documents.
- Maintain a change log of all changes in each version.
- Ensure only the latest approved version of a document is accessible.
- Customize approval workflows for various document types.
- Send document approval notifications and reminders.
- Capture comments and requested changes.
- Track training history for each document version.
Lockbox QMS Document Control provides your lab with a way to track, categorize, review, and approve controlled documents. This digital document control system helps labs achieve regulatory compliance and increase efficiency by maintaining document history, facilitating audit readiness, reducing errors and data loss, and automating approval processes.
QMS Document Control Features
The Lockbox QMS Document Control system allows users to track, categorize, note, and approve versions and revisions of Controlled Documents such as quality manuals, equipment calibration reports, and standard operating procedures (SOPs). Users can manage and store Controlled Documents, create new versions and route them for approval, capture comments and revisions throughout the approval process, and customize approval pathways. Together these features create a powerful and versatile electronic document management software that is easily connected to other Lockbox LIMS and QMS modules.
Managing Controlled Documents:
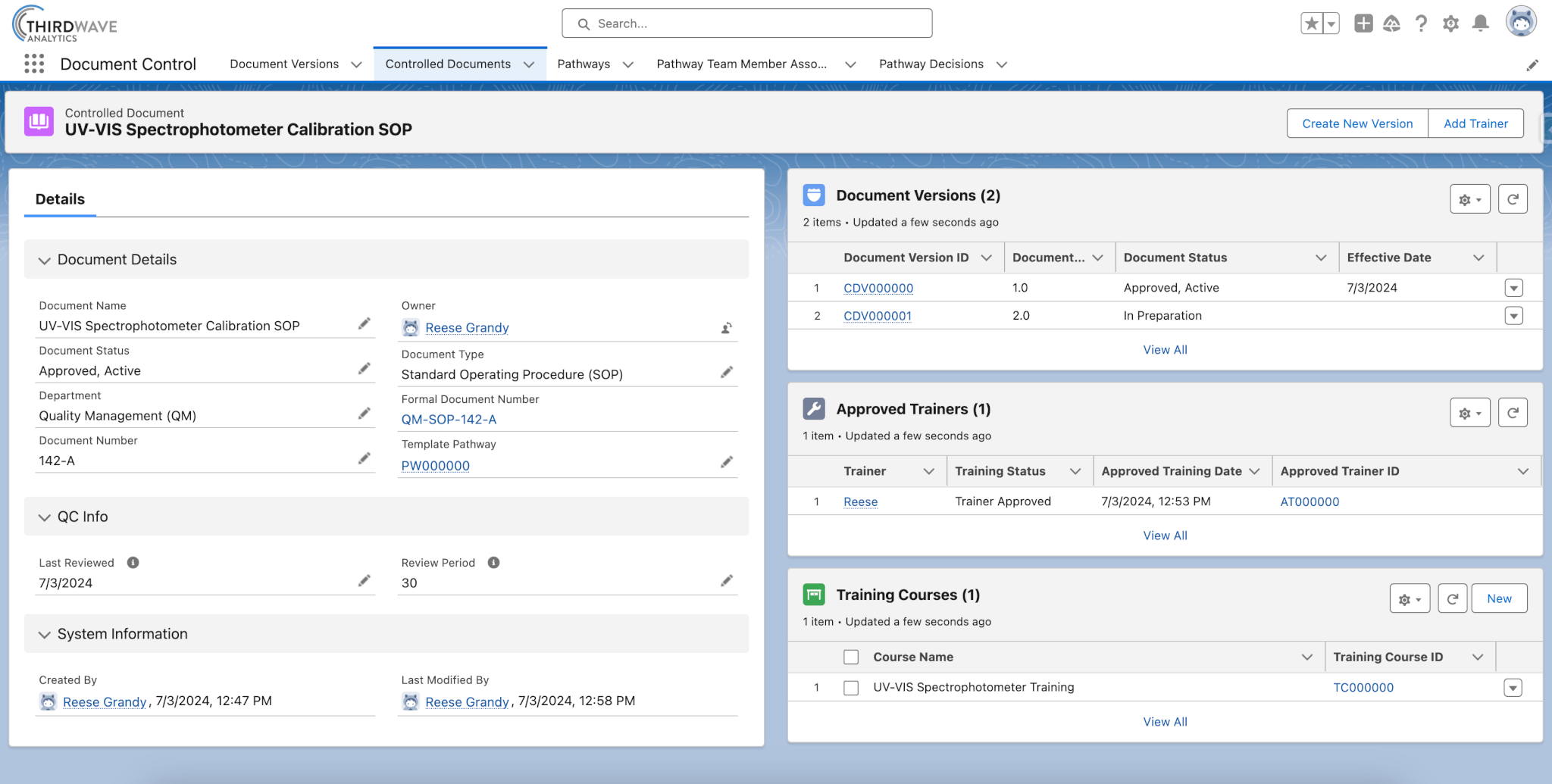
In Lockbox QMS, Controlled Documents can be managed in one centralized location. The overarching information about the document, including its name, document number, and approval status, is captured in the Controlled Document record. Associated to the record are new and historical versions, approved trainers, and training courses relevant to the Controlled Document.
- Provides a summary record that includes all Document Versions.
- Presents the purpose and uses of the document.
- Enables easy access to all versions of the Controlled Document.
- Associates approved Trainers and Training Courses to each Controlled Document.
Version History:
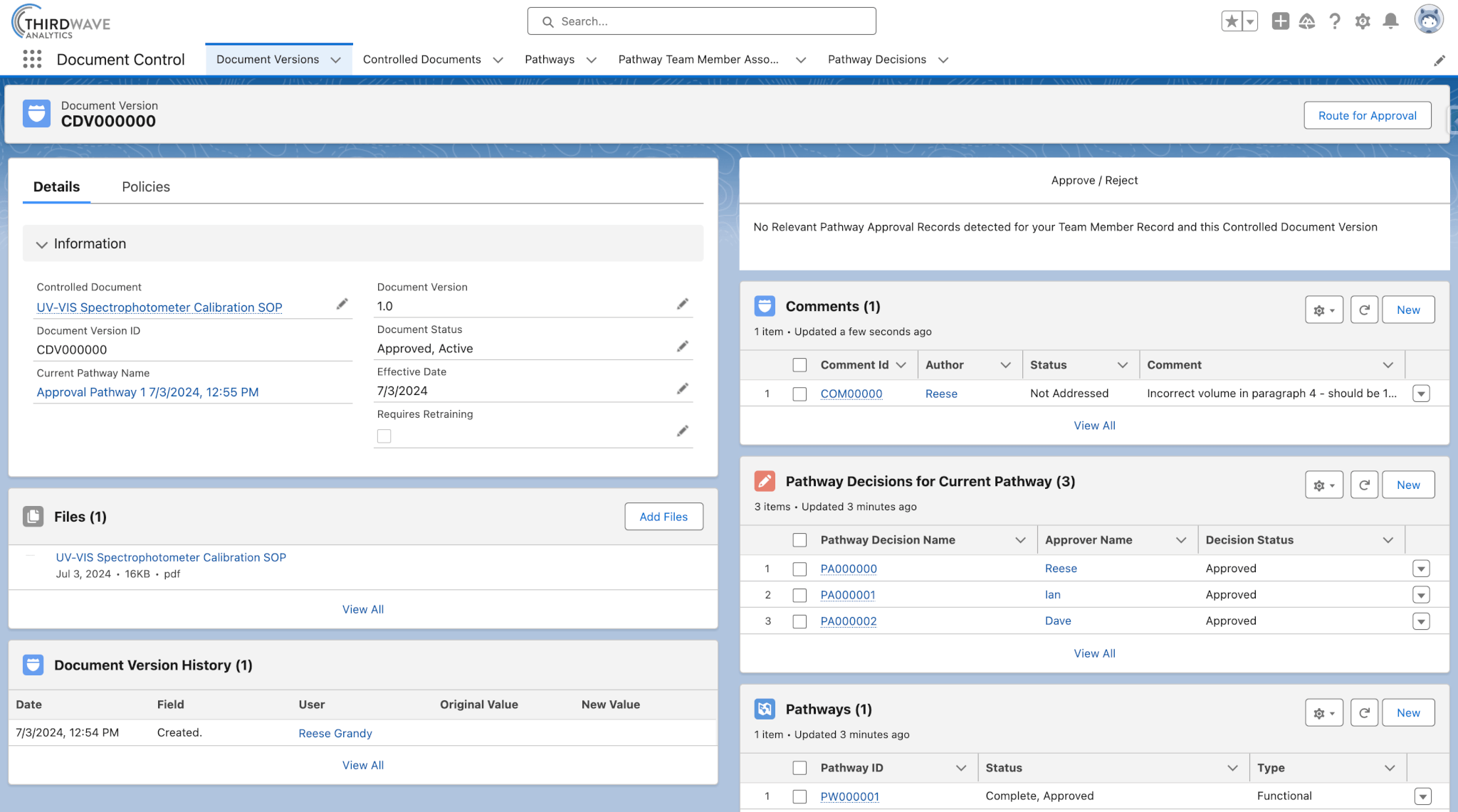
In Lockbox QMS, prior versions of a Controlled Document can be stored, maintained, and reviewed. As Controlled Documents are reviewed and revised over time, older versions are retired and new versions are activated. The current Document Version record of a Controlled Document supports additional features such as the ability to leave comments, request revisions, and create custom approval workflows.
- Each version of a Controlled Document has a Document Version record that stores revisions, comments, and approval history.
- The document is attached to this record as a file, providing easy access to Lockbox users with the appropriate permission.
- New Document Versions can be routed for review and approval.
- The user can easily view the approver and the approval status.
Access Control and Security:
Documents are securely stored in Lockbox QMS and protected by user-based permissions that determine who can view and modify documents. User permissions and role-based access can be configured to ensure only approved users are able to access current and archived Document Versions, revise documents, and approve changes.
- Configure permission sets and role-based access control to ensure only authorized users can access and modify controlled documents.
- Ensure only the latest approved version of a document is accessible.
- Utilize audit trails to track who has accessed and modified Controlled Documents and what changes were made.
- Maintain secure, centralized digital document storage.
Customizable Approval Workflows:
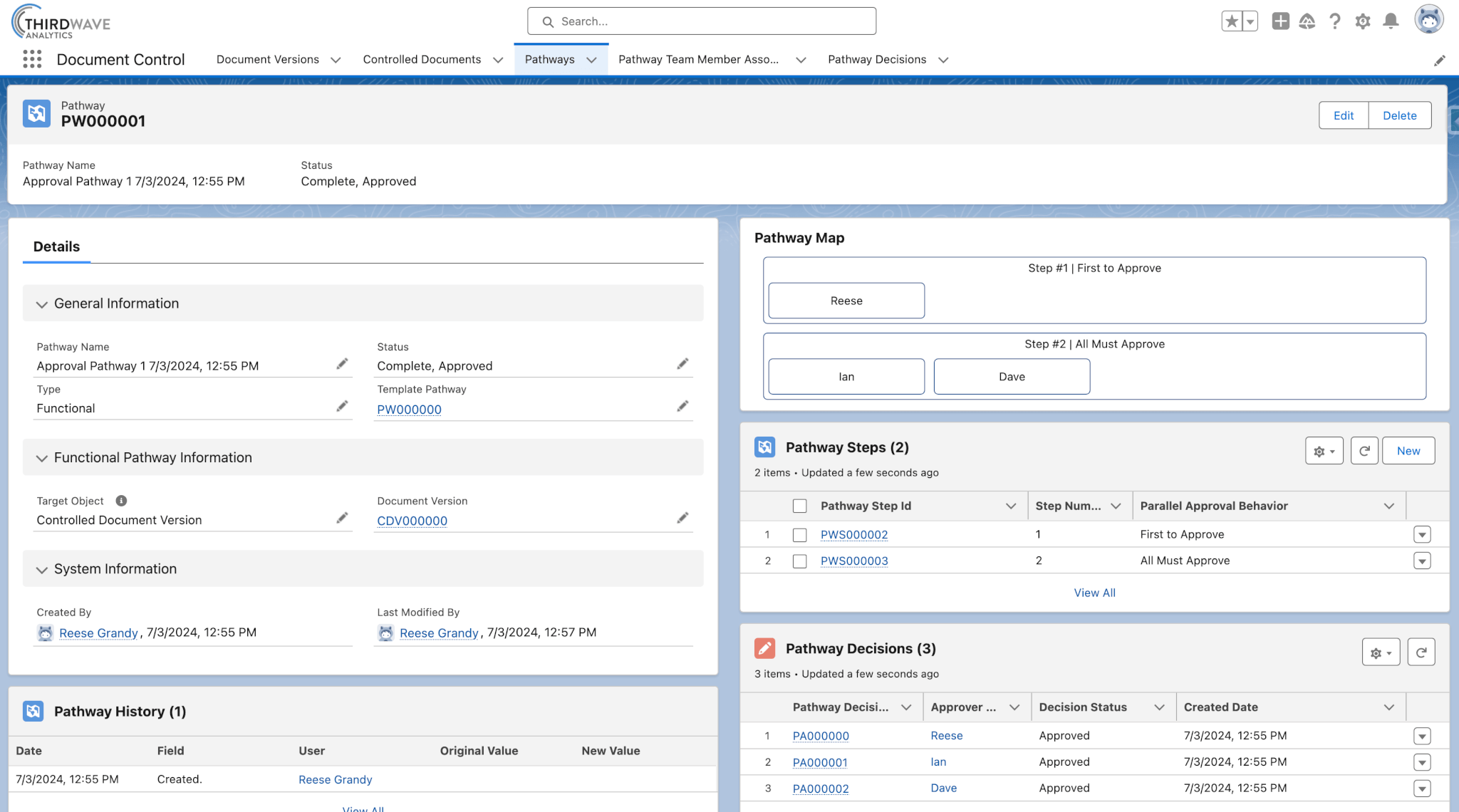
Controlled Documents can be routed through customized approval workflows, called Pathways, to ensure they are reviewed by the correct lab personnel before becoming active documents. The Pathway record includes an approval Pathway Map that visually displays who needs to approve the document and in what order. Each Pathway also maintains a historical record of approvals and rejections.
- Use the same approval workflow for every document or easily customize a Pathway for each document or document type.
- Easily see approval decisions and pending approvals.
- Automate approval reminders and routing to team members.
Capturing comments and revision notes:
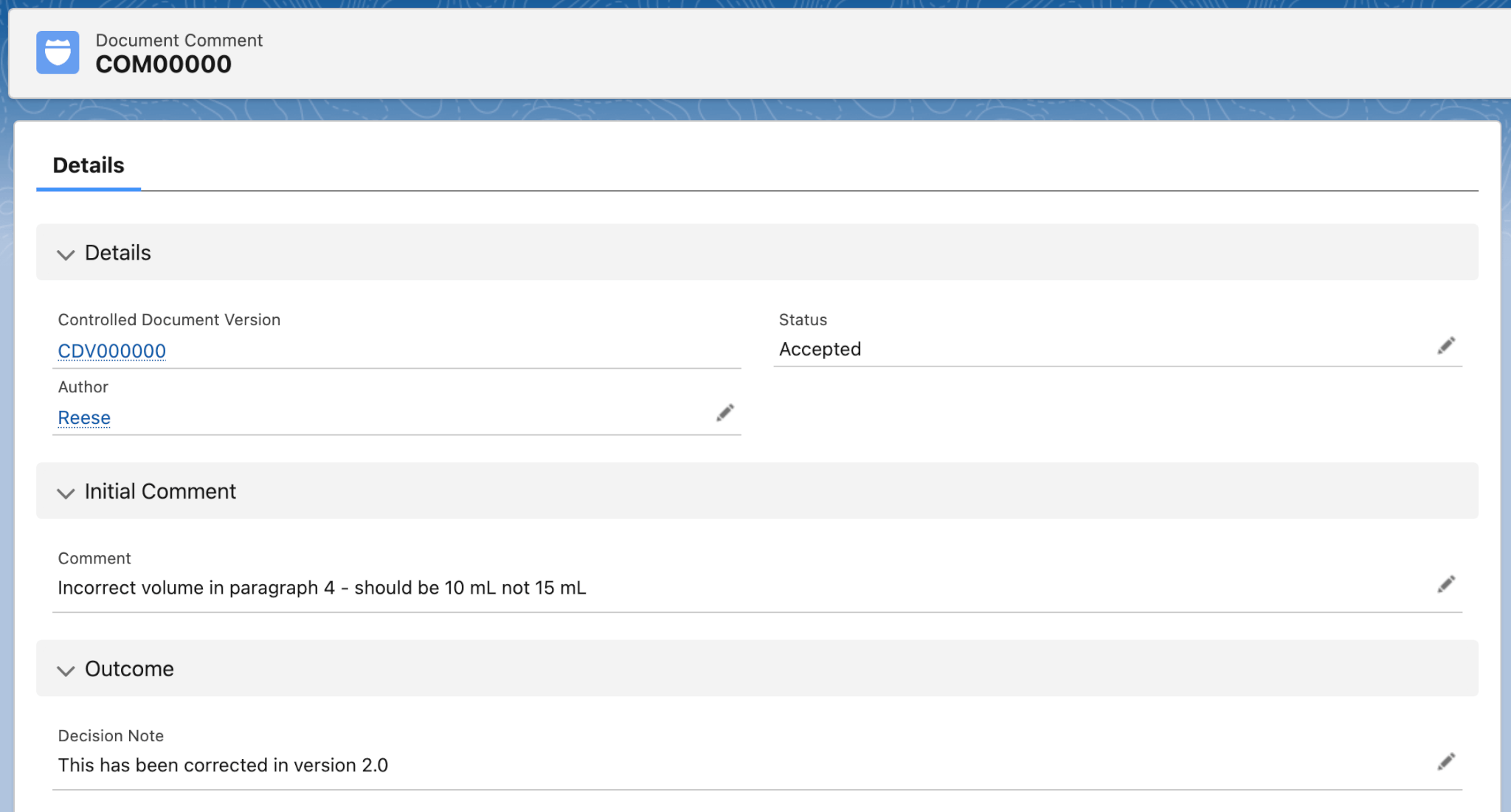
Users can leave Comments on Controlled Document records to highlight errors or updates that need to be addressed. These Comments can be accepted and applied to future versions of the Controlled Document.
- A simple record where users can add a note to an approved Controlled Document.
- Captures issues that need to be addressed at the next review.
- Captures the Comment author and status.
Centralized Document Repository:
Lockbox QMS Document Control provides a centralized document repository that allows you to store your Controlled Documents in a single digital space, saving you on-site storage space and making document retrieval easier and faster.
- Store all your quality documents in one location.
- Easily search for and retrieve documents.
- Categorize and tag documents for better organization.
Integration Capabilities:
Lockbox QMS can easily integrate with Lockbox LIMS to seamlessly connect your lab data with your quality documents.
- Lockbox LIMS customers can add Lockbox QMS modules to their existing Lockbox instance.
- Connect Lockbox LIMS objects including Samples, Protocols, Equipment, and Items to Lockbox QMS.
- A full suite of APIs is available for integrations with external systems.
- Provides CSV import/export functionalities for data migration.
Benefits of a Document Control System
Our Lockbox QMS users have appreciated the many benefits of the Document Control module. Some of the top benefits include:
- Quality documents are no longer siloed in a separate system from other lab and LIMS records. With Lockbox QMS, users can easily connect their lab data to their quality data.
- Documents are consolidated into a centralized digital repository, accessible anywhere there is internet access.
- Quality documents no longer live in paper binders or disorganized drives. Controlled Document digitalization and organization improves efficiency and accessibility, speeding up approval decision-making.
- This digital document control system is easily scalable and is designed to grow with your organization.
- Document Control helps companies achieve regulatory compliance and manage risk by maintaining document history and retention, facilitating audits, reducing errors and data loss, and helping to prevent security breaches.
Applications of Lockbox QMS Document Control
The potential applications of Lockbox QMS Document Control are endless. Here are a few examples of how our initial Lockbox QMS customers are using the Document Control module:
- Managing documents that pertain to or control various aspects of the laboratory and LIMS. These documents include standard operating procedures (SOPs), quality manuals, protocols and procedures, and equipment manuals.
- Managing project-specific documents, including contracts, permits, and non-disclosure agreements.
- Utilizing a Controlled Document Version to ensure that team members use the correct and most up-to-date documents.
- Ensuring document security by incorporating access controls and audit trails.
- Facilitating regulatory compliance audits with easy retrieval of old records, audit trails, and previous versions.
Ready to take the step and incorporate Lockbox QMS Document Control into your Lockbox instance? Contact your Engagement Director or our Sales Team to learn more.