Streamline CAPA Management
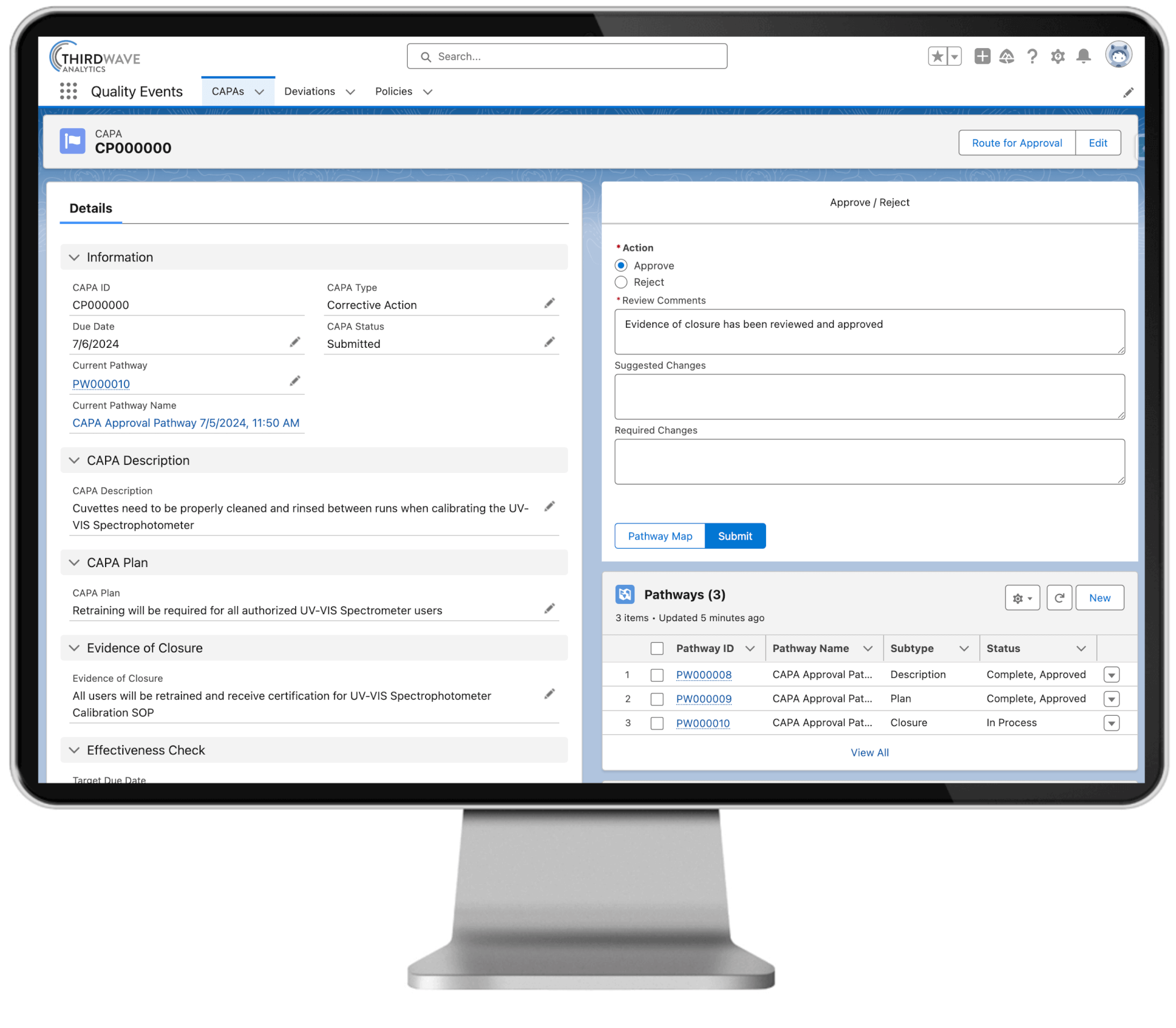
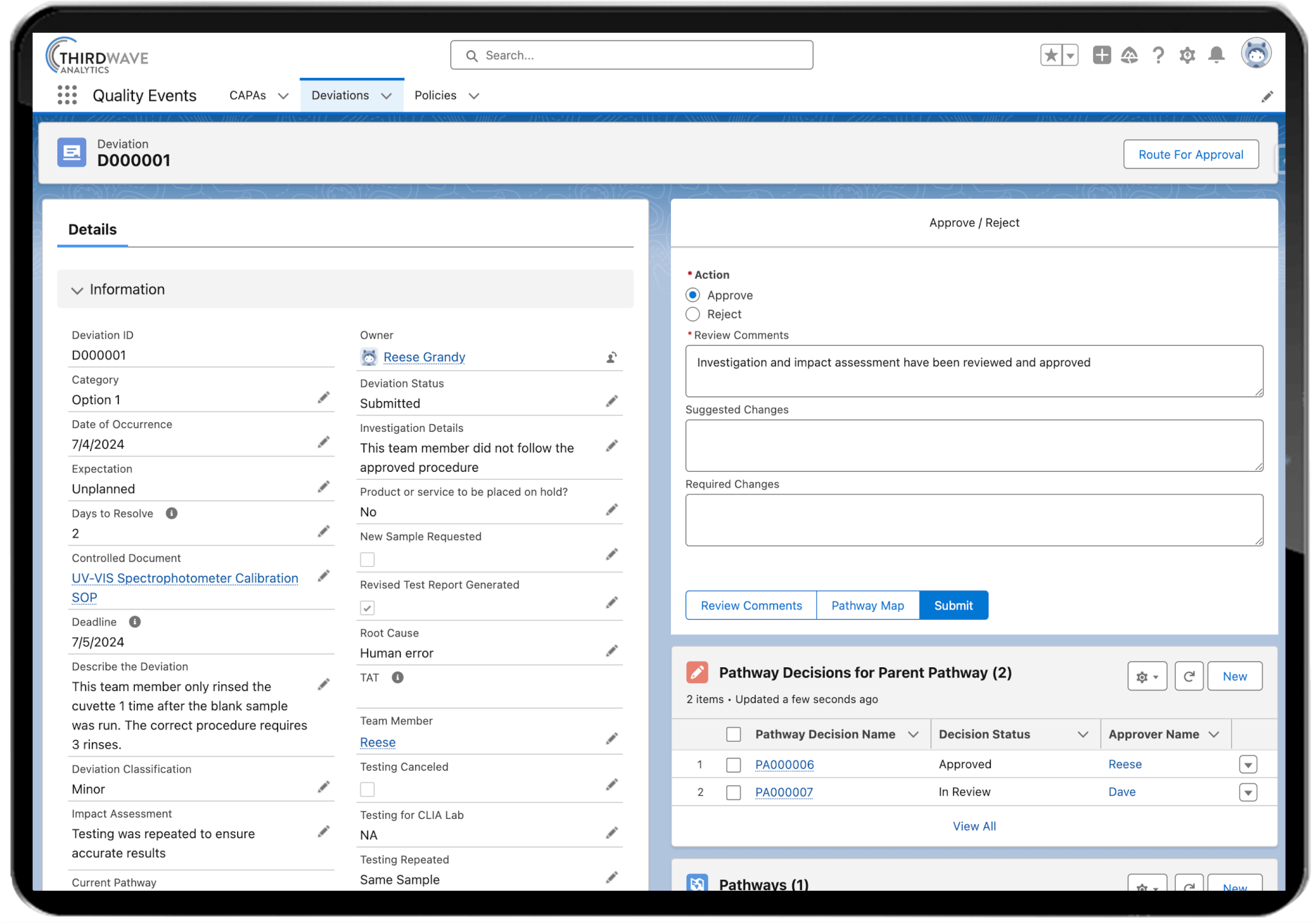
With Lockbox QMS, labs can track, investigate, review, and implement CAPAs, while tracking their effectiveness over time. The comprehensive reporting capabilities of Lockbox QMS enable users to see the status, progress, and resolution of quality events in real time.
- View open/closed status and track the time taken to address and close each quality event
- Track root causes and associate quality events to team members, procedures, samples, plates/batches, and reagents
- Record target due date and verification method to evaluate if the CAPA was effective
- Review and approve each component of a CAPA, including the description, plan, and closure
- Connect one or more deviations to a CAPA
- Automate and configure CAPA approval workflows
- Easily connect CAPAs to the Lockbox QMS Document Control and Training modules