Master Lab Compliance with Quality Event Tracking
Summary
The Lockbox QMS Quality Events module fulfills your laboratory’s need for a Deviation and CAPA quality event tracking system. This module allows users to:
- Report quality events, deviations, and CAPAs (Corrective and Preventive Actions) with ease.
- Monitor the status and progress of Quality Events in real-time.
- Assess the potential impact of Quality Events on operations and compliance.
- Connect Quality Events to team members, equipment, items, and samples.
- Maintain detailed records of all actions taken in response to Quality Events, including root cause analysis and effectiveness checks.
- Track key quality metrics including the number of Quality Events, time to close (TTC), and turnaround time (TAT).
Quality Event tracking is essential for maintaining regulatory compliance and ensuring consistent quality standards. Lockbox QMS Quality Events enables your lab to track how many Quality Events happened, when they occurred, and how long it took to close them. It facilitates evaluating and reporting on the samples, items, and equipment impacted by the Quality Event.
Deviations and CAPAs
The Lockbox QMS Quality Events module enables lab managers to keep track of two critical lab incidents: Deviations and CAPAs. In the laboratory setting, Deviations are planned or unplanned departures from approved processes, procedures, instructions, specifications, or established standards. CAPAs (Corrective And Preventive Action) provide a structure for discovering and resolving the root cause of problems. CAPAs also verify the solutions for future use and provide a framework for the systematic investigation of failure incidences.
Lockbox QMS Quality Events provides a mechanism to log and track Deviations and CAPAs, and maintain a record of the solutions that laboratories implement. By maintaining comprehensive records and audit trails, Lockbox QMS enables smooth internal and external audits for laboratories working to comply with various regulatory standards, such as FDA, CAP/CLIA, and ISO.
Incident Reporting and Tracking
To meet regulatory requirements, laboratories regulated by agencies like the FDA or working under established domestic and international standards like CAP/CLIA and ISO require comprehensive incident reporting with a detailed record of incidents and resolutions. The comprehensive tracking and reporting capabilities of Lockbox QMS Quality Events help streamline the process of managing Deviations and CAPAs and provide visibility of the status, progress, and resolution of Quality Events.
- Track the time required to resolve Deviations and CAPAs.
- View open/closed status and track the time taken to address and close each Quality Event.
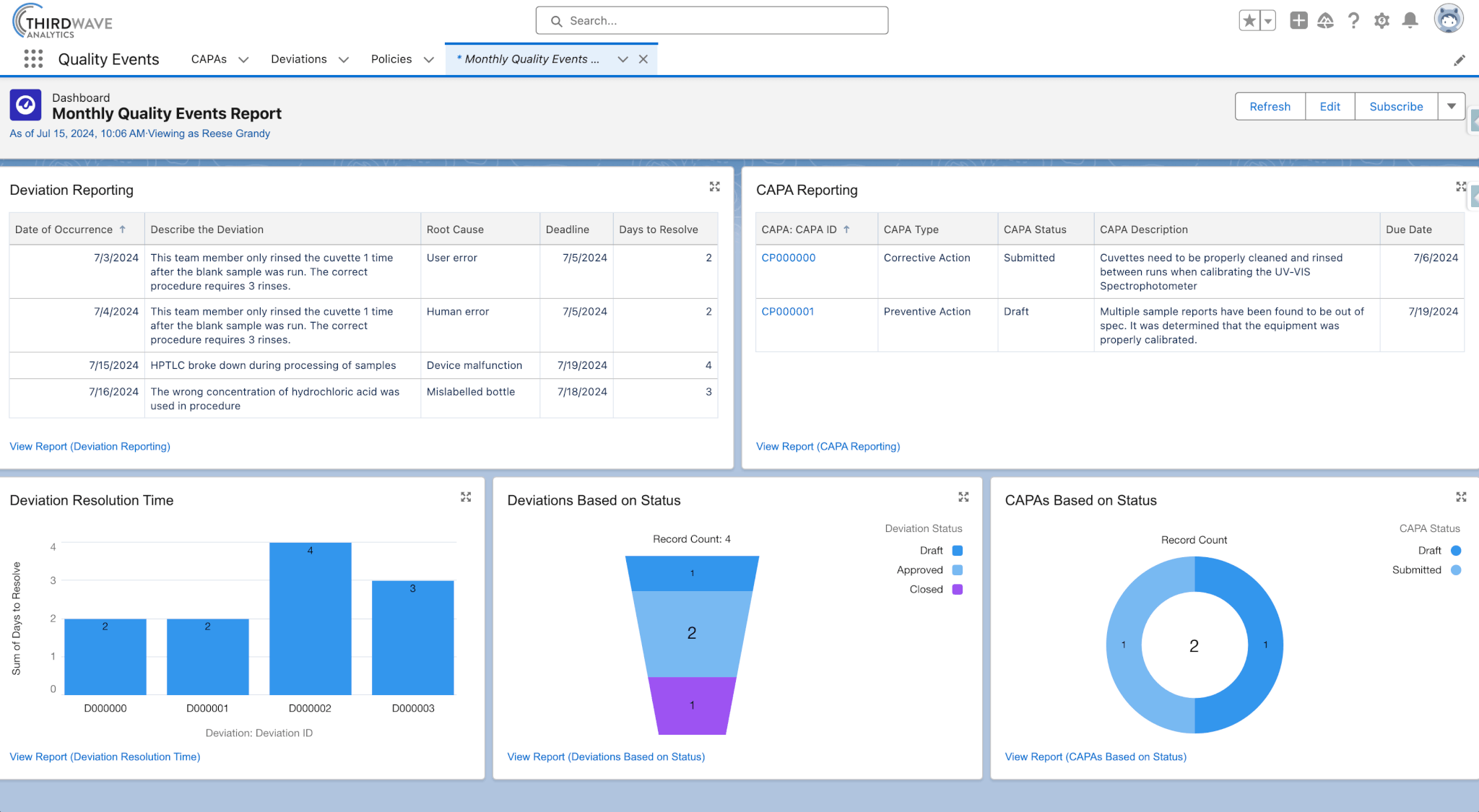
- Report on the number of Quality Events over a specific period.
- Track root causes and associate Quality Events to team members, procedures, samples, and reagents.
- Use effectiveness checks to measure the success of CAPAs in preventing the recurrence of Quality Events.
Quality Event Approval Workflows
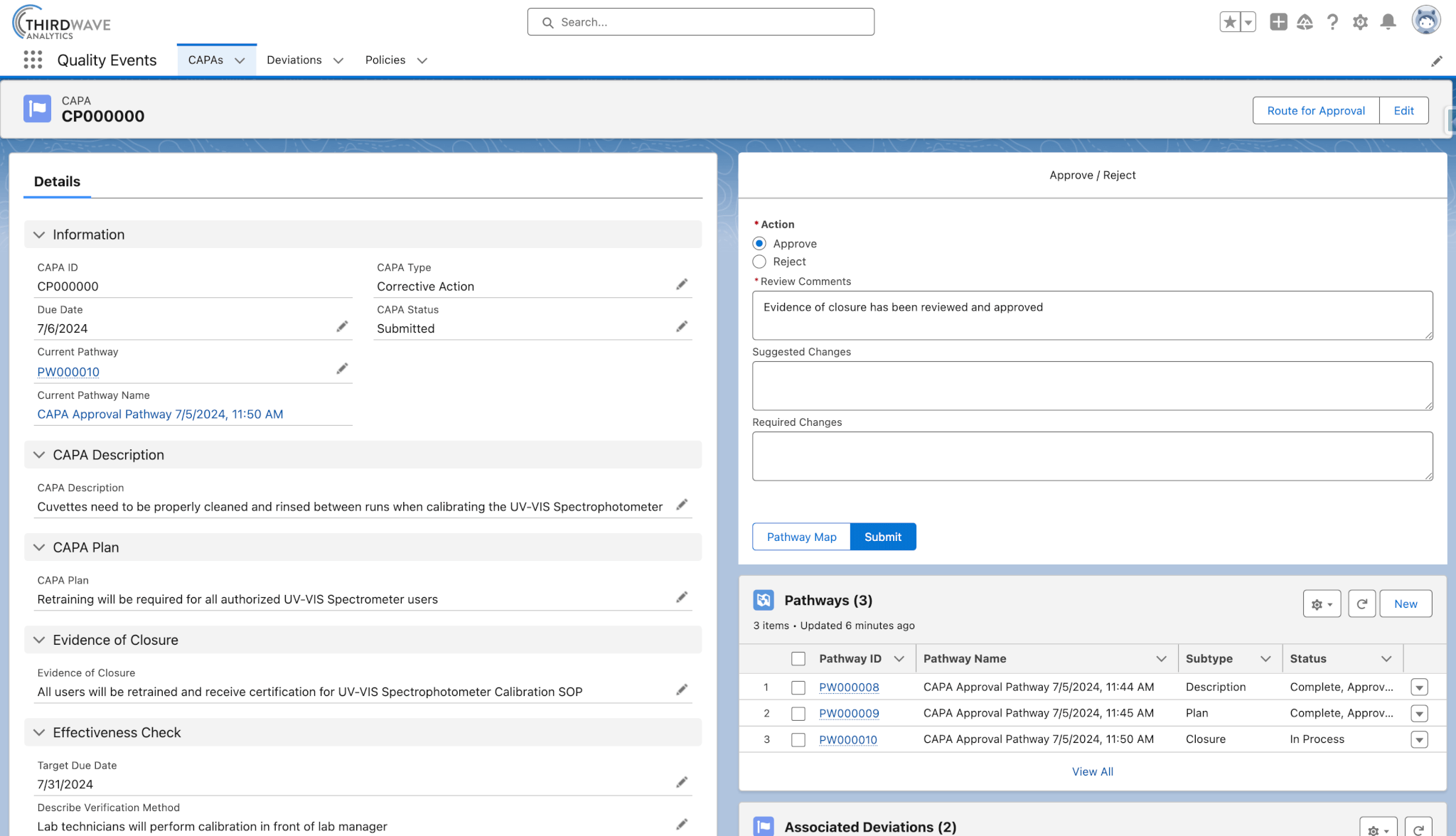
Routing Quality Events through approval workflows is essential for ensuring thorough evaluation, proper documentation, and effective resolution. In Lockbox QMS you can customize your lab’s approval process so that key members of your team can review, comment on, and approve Quality Event records.
- Automatically route deviations and CAPAs to the appropriate lab personnel for approval.
- Quality event statuses are automatically updated based on approvals received.
- Review and approve each component of a CAPA, including the description, plan, and closure.
- Automatically notify the team members responsible for reviewing Quality Events.
- Trigger automations upon approval/rejection.
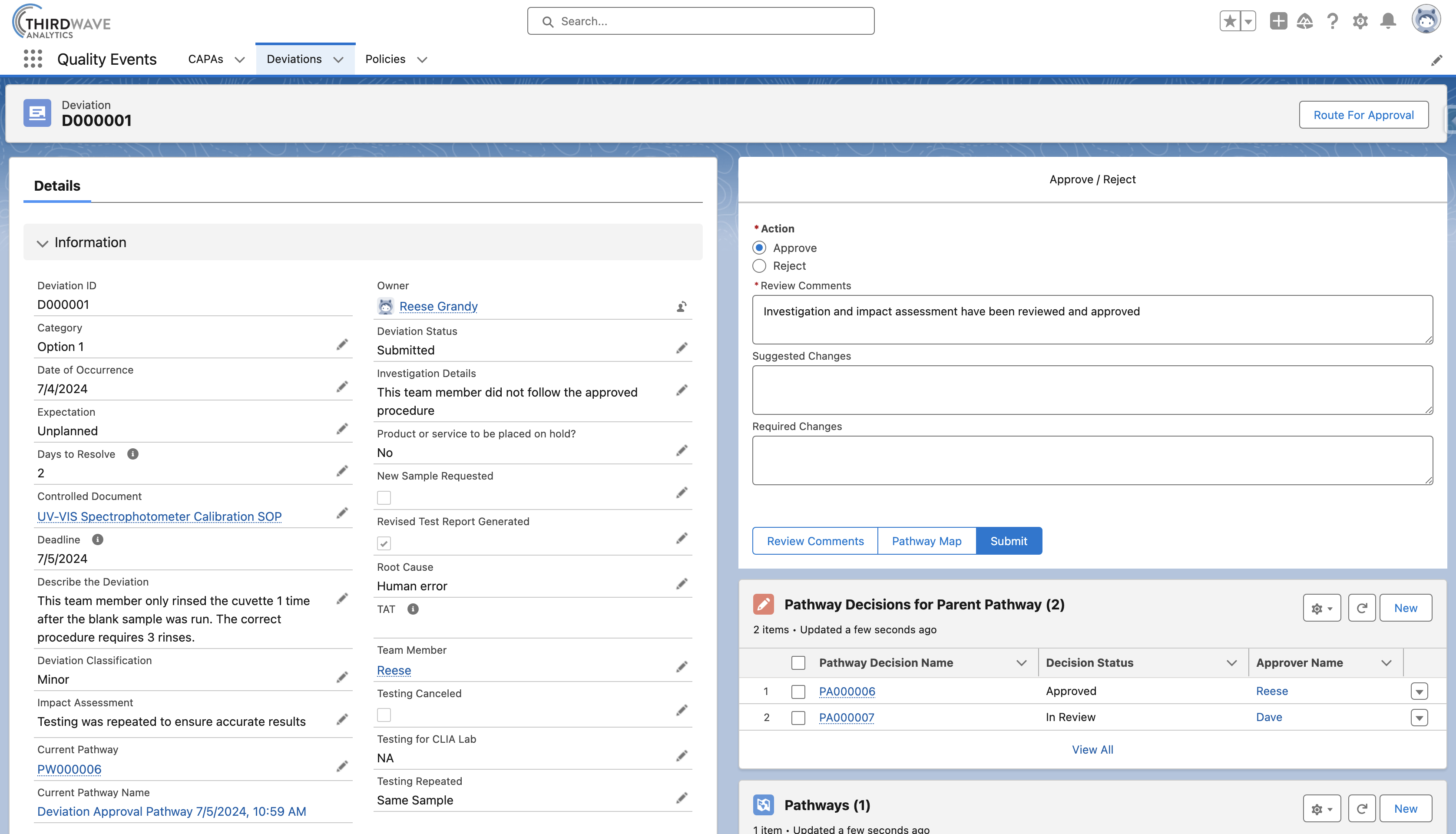
Root Cause Analysis
Finding the root cause of a Deviation is crucial for preventing recurrence, improving processes, and ensuring compliance. Lockbox QMS Quality Events gives users the ability to track Deviations and their root causes, as well as associate those Deviations to a CAPA.
- Link one or more Deviations to a CAPA.
- Capture investigation details and root causes.
- Perform impact assessments and record the effect on testing and reporting.
- View team members, procedures, samples, and reagents associated with Quality Events.
Regulatory Compliance
Organizations are often required to track and report on Quality Events as part of their compliance with standards such as 21 CFR Part 11 & Part 820, CAP/CLIA, and ISO 17025 & 13485. Lockbox QMS Quality Events maintains detailed records of all actions taken in response to a Quality Event, including the date of the event, a description of the event, the personnel involved, and any impacts or follow-up actions.
- Use audit trails to store detailed records of all actions taken in response to Quality Events.
- Update SOPs or other Controlled Documents in response to a Deviation and/or CAPA.
- Automatically generate reports to facilitate smooth internal and external audits.
- Tracks key quality metrics to evaluate the effectiveness of CAPAs.
- Maintain comprehensive documentation of all Quality Events in a centralized, searchable repository.
Pre-integrated with Lockbox LIMS
Lockbox QMS comes pre-integrated with Lockbox LIMS, allowing you to add your Quality Management System directly to your existing Lockbox LIMS system.
- Connect Lockbox LIMS features including Samples, Protocols, Equipment, and Items to Lockbox QMS.
- A full suite of APIs is available for integrations with external systems.
- Provides CSV import/export functionalities for data migration.
Customization
Lockbox QMS is built on the same platform as Lockbox LIMS, providing all the same point-and-click customization options that you appreciate in Lockbox LIMS. Nearly every part of Lockbox QMS can be customized to meet your lab’s specific needs, including page layouts, fields, automated reminders, and relationships to other Lockbox functions. For example, some customers have customized the Lockbox QMS Quality Events feature to track “occurrences”. For these labs, an occurrence is a special type of deviation that happens before or after the analytical steps. With Lockbox QMS, this tracking feature can be added with no vendor support in a matter of hours. Your Lockbox QMS Quality Events module can also be customized to include change control, giving you a systematic approach to managing product, process, or system changes.
Applications of Lockbox QMS
The many features of Lockbox QMS Quality Events provide an unlimited array of possible applications. One application that customers have found valuable is the ability to monitor how long it takes to close out deviations. Another is the CAPA effectiveness check, which enables users to see the impact of a CAPA and determine whether a further change in process is needed.
When your laboratory adopts both Lockbox LIMS and Lockbox QMS as one unified system, there is a huge value-add to your productivity. Employees can quickly, easily, and automatically evaluate the impact of Deviations and CAPAs on Samples, Plates/Batches, Inventory, Tests, Protocols/Procedures, Storage, and Equipment. This reduces the time from Quality Event occurrence to decision. Reporting Deviations and CAPAs makes it very easy for laboratory directors and all other users to quickly see how many Quality Events happened, when they occurred, how long it took to close them, the closed dates, how many events are currently open and closed, and what Samples or Equipment are affected.
Lockbox QMS Security Features
Your Third Wave Analytics implementation team will work with you to set up your team members’ access model in Lockbox QMS. User groups and permission sets will ensure that only designated users can view, log, approve, or report on a CAPA or deviation. Lockbox QMS is built on the Salesforce platform and comes with all the security features Salesforce and Lockbox LIMS have to offer.
Ready to take the step and incorporate Lockbox QMS Quality Events into your Lockbox instance? Contact your Engagement Director or our Sales Team to learn more.