How Lockbox Can Help You Meet CAP/CLIA Regulatory Requirements
Summary
CAP/CLIA laboratories follow United States federal regulatory standards set by the College of American Pathologists (CAP) and the Clinical Laboratory Improvement Amendment (CLIA). This applies to laboratories performing diagnostic tests on human samples in the United States. European laboratories often follow ISO15189, which incorporates several elements of CAP/CLIA but is not a substitute.
This article highlights Lockbox LIMS features that help laboratories attain CAP/CLIA certification. Using a LIMS is a facility-driven determination, but the benefits of using Lockbox LIMS include retiring labor-intensive Excel documentation, improving your workflows, and helping your lab meet CAP/CLIA requirements.
Audit trails
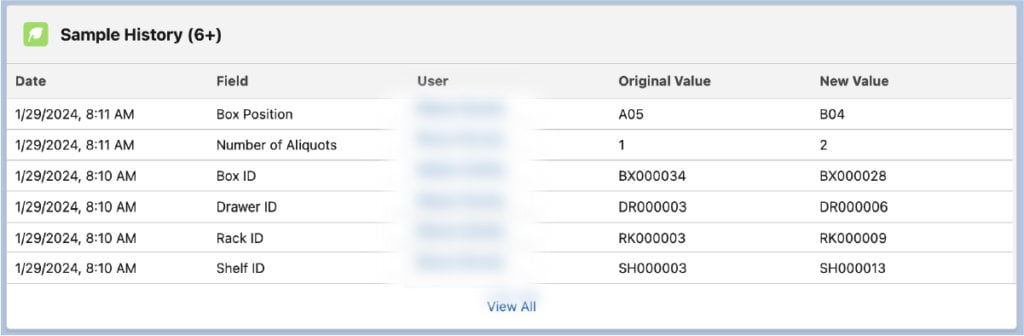
Lockbox has built-in field audit trails that allow users to track up to 60 fields per Object, allowing users to meet data integrity and traceability requirements. The field history is stored indefinitely or deleted after a specific number of years that meets your organization’s data retention policy. The information that can be tracked at the field level is as follows and depicted below:
- Date/time change is made
- The field that was changed
- Who made the change
- What it changed from
- What it changed to
21 CFR Part 11
Lockbox supports electronic signatures for laboratories to maintain the United States 21 CFR Part 11 and EU Annex 11 compliance, discussed here. Lockbox can be configured so that users must enter a username and password at critical points to sign off on results or reports. Lockbox can also integrate with third-party document signing software such as Docusign.
Password Requirements
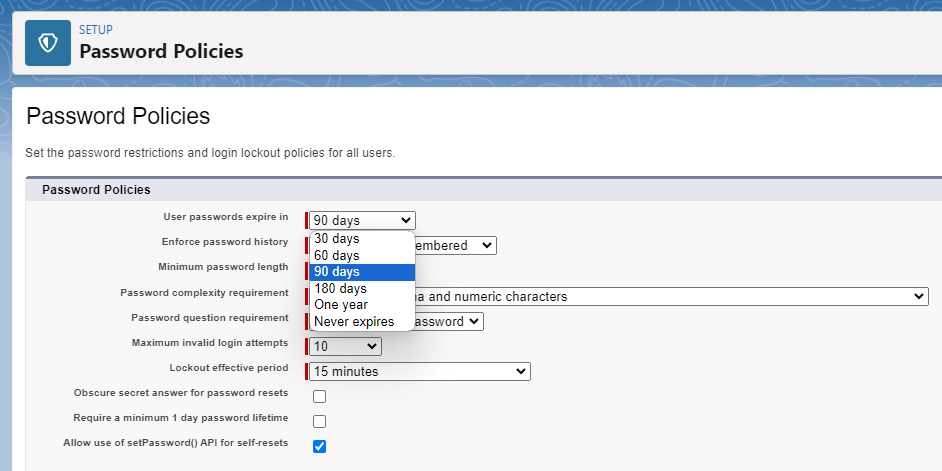
Lockbox takes security seriously. For a deep dive into Lockbox security features, please see The Importance of Security in LIMS Data Management. Lockbox user passwords must have appropriate complexity and changed at an interval set by the admin.
Shield/Encryption
Clinical laboratories frequently interact with personal health information (PHI). Lockbox can meet nearly all global privacy standards for securing PHI and personal identifiable information (PII). Lockbox is built on the Salesforce Platform, which allows your organization to benefit from comprehensive Salesforce security and encryption features. For instance, Salesforce Shield provides advanced encryption options. To learn more about Salesforce Shield encryption capabilities and privacy standards that Lockbox incorporates, please see Meet Your Lab’s Regulatory Compliance Requirements With Lockbox LIMS.
Backup/Data Retention
To meet CAP/CLIA requirements, LIMS must be backed up at regular intervals. Data must be kept for at least 7 years, or longer in some states. Salesforce Shield meets the requirements for data retention of field audit trails with the ability to retain data for up to 10 years. Salesforce also provides a secure infrastructure to keep your data secure and accessible with highly redundant servers. Users can export data in a CSV format at regular intervals, and purchase Salesforce Backup for high-volume backup requirements. Please speak with your Third Wave Analytics Engagement Director to learn more.
Profiles/Permissions/Sharing
Lockbox user profiles, permissions, and sharing rules are incredibly agile and can be configured to allow or prevent internal and/or external user access to data. We discuss profile and permission sets in detail in The Importance of Security in LIMS Data Management. Your IT or admin team can configure profiles and permission sets to ensure:
- Only the appropriate personnel have access to PHI.
- Only appropriate personnel have access to view/edit/create/delete records.
- Only the appropriate personnel can enter test results (for instance, the clinical laboratory scientist or physician signing out reports).
- That clients logging in through a portal only have access to their patient records and can only see appropriate information.
Result Verification
Lockbox can be configured to interpret test results and produce a report. Using Lockbox for this purpose saves clinicians time and eliminates human reporting errors. If your laboratory uses Lockbox in this way. your Lockbox org will need verification and validation. Your implementation team can help you in this process.
Lockbox LIMS training module
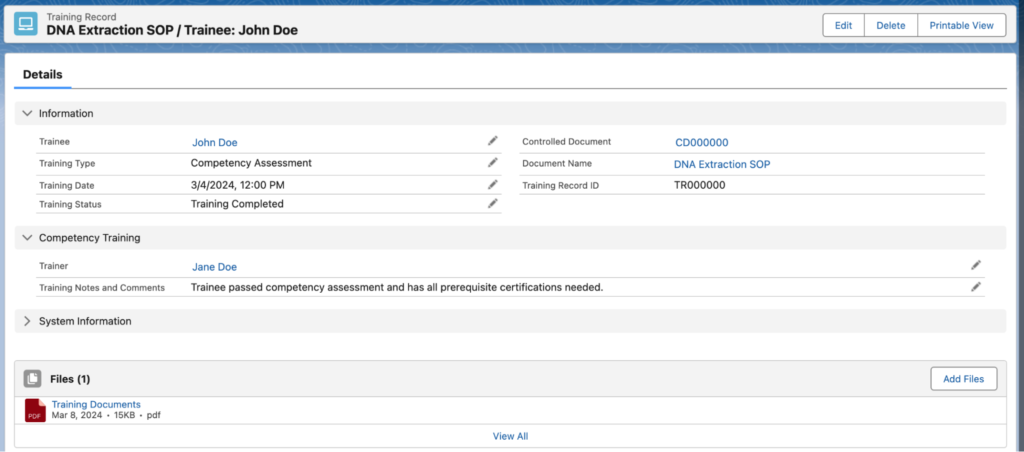
In CAP/CLIA certified laboratories technicians must be properly trained to execute protocols or use equipment. The Lockbox LIMS training module allows users to track an individual’s training, shown below. Lockbox can prevent a user from executing a protocol or reserving equipment if they haven’t completed required training.
Inventory Management Module
The Lockbox LIMS Inventory Management Module is an essential asset to CAP/CLIA laboratories. The inventory management module allows users to track critical reagent information such as Lot and/or Expiration Date in Protocol Execution and see which samples were processed with that lot. Lockbox LIMS reporting capabilities are critical in inventory management because if a bad lot is identified users can easily run a report to identify any impacted sample. To see how the inventory management module works please see our video demonstration.
Protocol Versioning
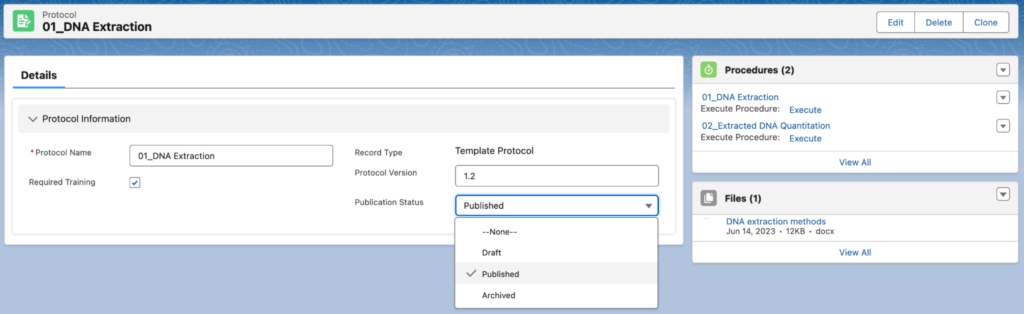
Lockbox LIMS Protocol objects allow users to version Protocols when changes are made. Users can assign a version number and a publication status appropriate for their laboratory (as seen below). The previously used protocols do not change and remain linked to samples processed under that version. This allows users to retain traceability.
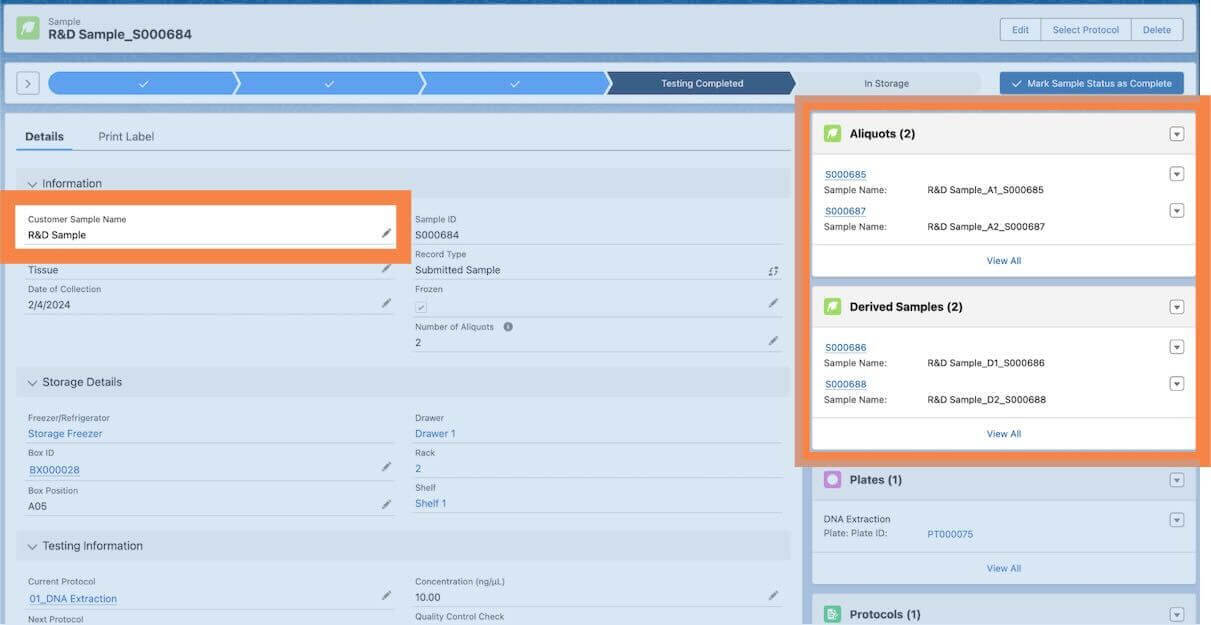
Sample hierarchy tracking
Lockbox LIMS also has powerful sample lineage tracking built in, which enables users to create submitted, derived, and aliquot samples and easily identify parentage/child samples through Related Lists and Sample Naming, shown below.
Conclusion
These Lockbox LIMS features support your lab in meeting CAP/CLIA requirements. To learn how Lockbox can help your lab meet other laboratory regulations and compliance standards like HIPAA and 21 CFR Part 11, check out our complete review on regulatory compliance.
If you have any additional questions on how Lockbox LIMS can meet your lab compliance management needs don’t hesitate to contact us! Our experts are here to answer all of your questions.