What is a Modern LIMS?
Watch now: What is a LIMS?
Let’s start by answering the question, ‘What is a LIMS?’ and then dive into its core functions.
LIMS stands for Laboratory Information Management System. Often called a ‘LIMS System’ or ‘LIMS software,’ it functions similarly to other data management systems used throughout the business world.
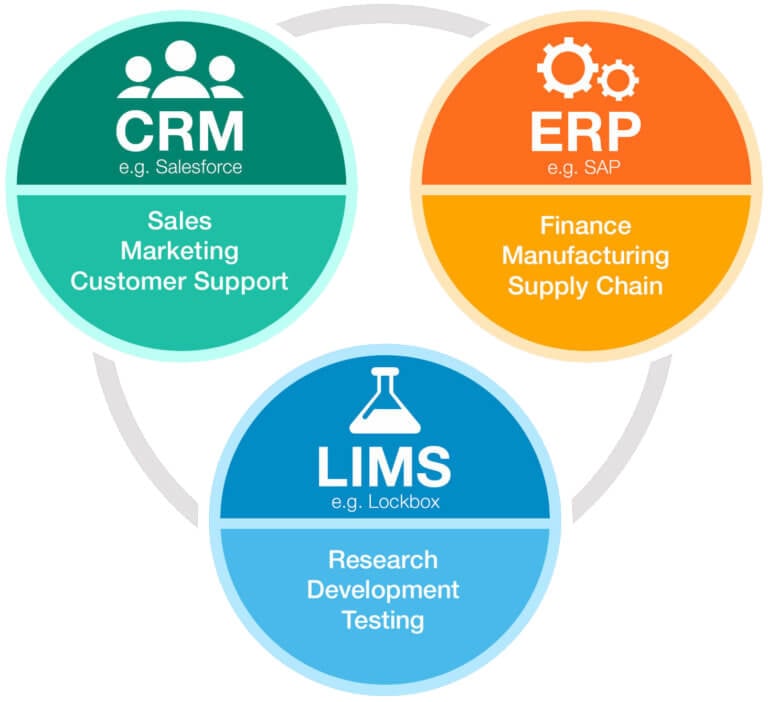
These software applications are usually implemented to manage and automate previously manual, fragmented, or inefficient processes. For example, customer-facing sales, marketing, and customer support departments may use a customer relationship management (CRM) application like Salesforce or Microsoft Dynamics to automate ongoing transactions, accounts, and customer communication. At the same time, the company’s finance, human resources, and manufacturing departments might use enterprise resource planning (ERP) software like SAP or Oracle for workflow automation, data integrity, regulatory compliance, and increased operational efficiency.
Likewise, research and development (R&D) departments also have software applications that address their specific needs to streamline and manage product research, development, and testing. This department type will also likely require precise data handling — and quality control, traceability, and audit trails required for regulatory compliance. The software that handles this process is called a Laboratory Information Management System, or LIMS solution. It is also sometimes called a Laboratory Information System (LIS) or Laboratory Management System.
Laboratory Information Management Systems are widely used throughout organizations specializing in R&D, such as universities, contract research organizations (CROs), biobanks, and clinical research facilities. All these organizations need high-quality sample management and laboratory data that can be accessed and analyzed for real-time validation.
With that outline describing how a LIMS fits into an organization’s framework, we’ll take a closer look at:
- What a LIMS does
- The core components of a LIMS
- The main benefits of a LIMS
- How a LIMS differs from an Electronic Laboratory Notebook (ELN)
- When to consider LIMS implementation for your organization
- Which LIMS products are highly rated
What does a LIMS system do?
A comprehensive LIMS supports crucial aspects of today’s complex laboratory processes. When used across various industries and research institutions, a LIMS should accurately manage and distill vast amounts of data so researchers can deliver more significant insights faster. As a major part of an organization’s laboratory informatics, a LIMS improves operations by managing data related to samples, experiments, workflows, and instruments. It also digitally records and tracks metadata, results, workflows, and instruments associated with lab samples.
At the heart of it, a LIMS provides a system to track, standardize, organize, and centralize all the data, processes, and tasks in a lab. A LIMS is in essence, a database – but, in operation, it does a lot more than a database. Below, we’ll dig into the details.
The core components of a LIMS
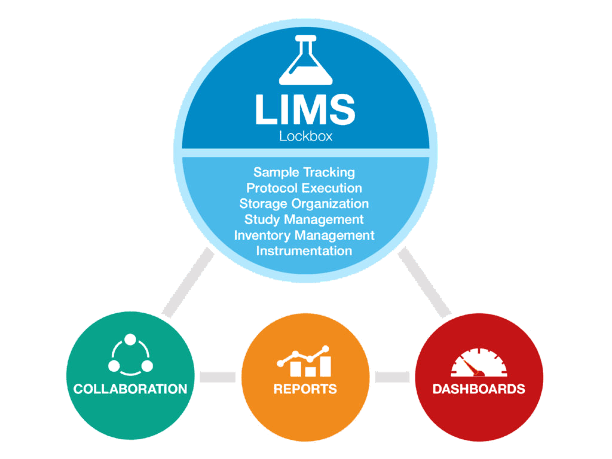
A good LIMS has three main components: Sample Tracking, Protocol Execution, and Storage Organization. Imagine a lab in which samples are tracked differently by different researchers, using methods varying between a pen and paper and a massive spreadsheet. It would be extremely difficult to ensure the data isn’t compromised by human error — such as missing results, errors, and differences in data collected that can compound any mistake.
Below, we examine the three components of a LIMS and explain how they benefit researchers and lab managers.
Sample Tracking
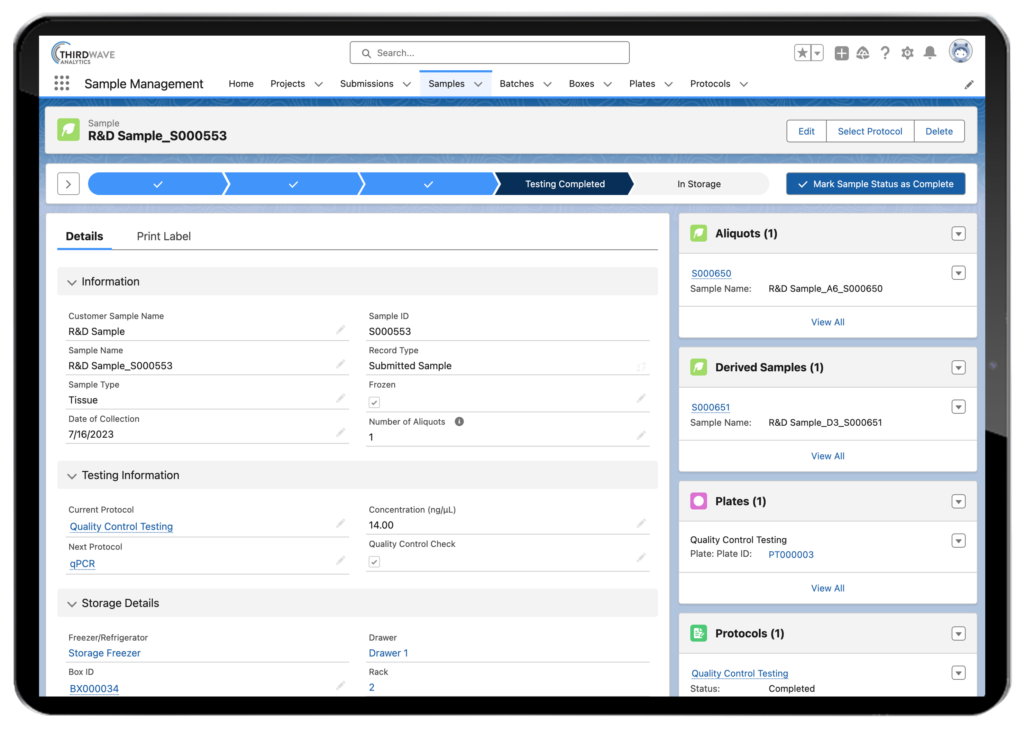
The primary function of a LIMS is to track a sample from the time it arrives in a laboratory through its testing and storage. This includes recording all data associated with the sample upon its initial accessions, such as the sample’s ID, source, collection date, and quantitation information (i.e., concentration, volume, and particulate amount). As the lab sample progresses along its workflow, additional data is captured and stored in the LIMS. This includes test results, derived sample data, and time-based study metrics.
In addition to capturing and tracking data specific to each lab sample, a LIMS also tracks who interacted with a sample and where it was throughout its lifecycle. For example, a sample may be placed in a batch or pooled with other samples the laboratory is testing. An external barcode label must track the lab sample affixed to the test tube and a chemical ‘index.’ This allows it to be identified from the other samples in the pool. All of this valuable sample tracking information is stored in the LIMS system. Sample data storage is only one component of a LIMS, and therefore answers only one part of the question What is LIMS in a lab? LIMS software is also critical for developing a lab’s workflows and protocols. Please take a look at our video showing the Lockbox LIMS Sample Tracking Module.
Protocol Execution
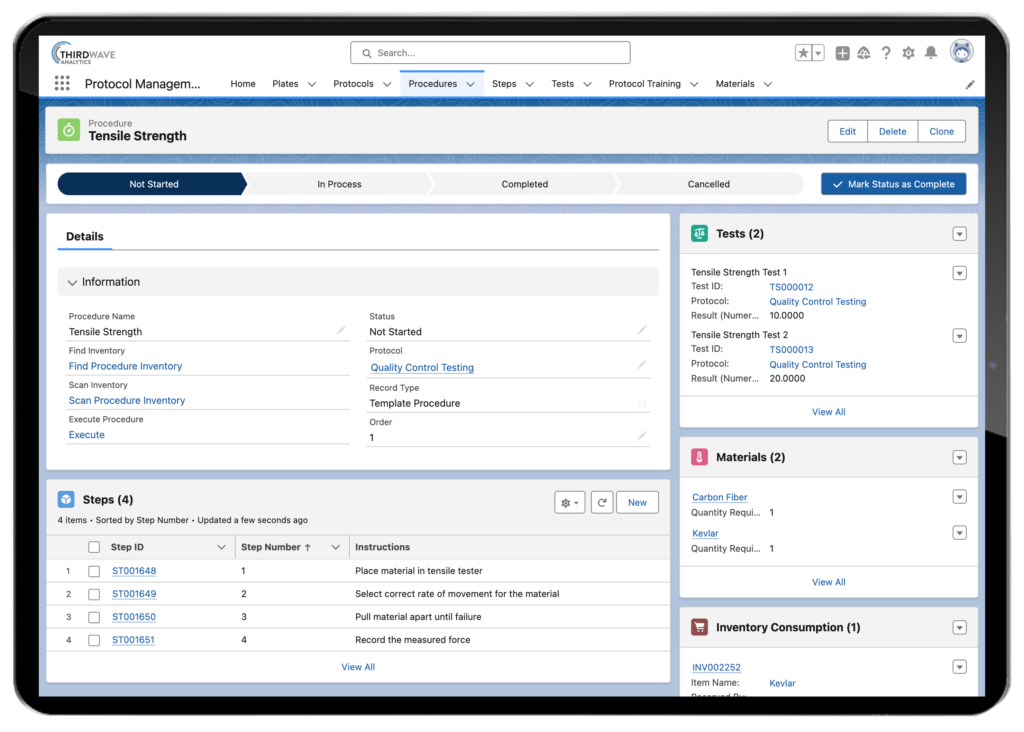
The second primary function of laboratory information management system software is to drive the standardization of a lab’s workflows and underlying protocols, procedures, and steps. Ensuring that each lab tech adheres to the specific steps in a published SOP (Standard Operating Procedure) when processing a sample, regardless of who is processing the sample or running a test, is critical to obtaining an accurate and repeatable result. A LIMS supports standardization across the laboratory team by digitizing the steps in procedures and protocols. This ensures the entire lab staff executes the correct steps in the correct order when running a sample through a test.
LIMS software can manage test assignments so that when a sample arrives in a laboratory, it is immediately assigned the appropriate protocol. A LIMS can also provide a lab with more stringent protocol version control measures, granting visibility to research or clinical teams, depending on who is authorized to run the protocol. Lab test results can be recorded, sent through the appropriate approval queue, and distributed to the necessary team members via reports. Please take a look at our video showing the Lockbox LIMS Protocol Management Module.
Storage Organization
The third critical feature in a Laboratory Information Management System is keeping track of where a sample is throughout its laboratory lifecycle. Starting with the individual lab sample, the LIMS tracks where, in a particular box, the sample tube or vial is kept (for example, slot A1 or B5). Next, the system keeps track of which drawer that box is in and which rack the drawer is in. Furthermore, the system tracks which shelf the rack is on and which room the freezer is in.
This “storage hierarchy” (Sample > Position > Box > Drawer > Rack > Shelf > Freezer > Room) plays a critical role in locating samples quickly in busy laboratories. Research teams that know precisely where lab samples are stay productive, organized, and efficient. Please take a look at our video showing the Lockbox LIMS Storage Management Module.
The main benefits of a LIMS
As a software solution, LIMS deliver a myriad of benefits for busy lab managers and staff:
- Data Centralization. One of the most significant benefits of LIMS software is having a centralized system to manage all the peripheral components of a lab utilized in executing a workflow. (This is a benefit whether you’re using a LIMS system for small laboratories or enterprise-size facilities.) Information about studies, instrumentation, and inventory can be stored in one centralized location, easily accessible to the lab team when needed.
- Study Management. Lab samples are typically collected supporting a higher-level research study or project. A LIMS provides the organizational structure to group related samples together underneath the appropriate study or project. These studies typically have subjects associated with them (human or otherwise), which can be grouped into cohorts with associated visits. A fully functional LIMS software solution allows researchers to manage all this information in a single system, thus centralizing all the information for more robust data management.
- Inventory Management. Integral to any lab’s ongoing operations is its reliance on lab supplies, such as reagents and chemical inventory. A laboratory’s staff must have the necessary inventory to execute protocols effectively. A LIMS is a vital tool to provide the lab with insight into what material is on hand and what might need to be ordered. As lab staff use supplies, a LIMS can automatically mark the supplies as consumed and trigger a re-order process. This limits the potential for error or delay and improves a team’s efficiency. A LIMS also provides lot number tracking so the specific reagent used to process a lab sample is recorded in the system for future audit purposes.
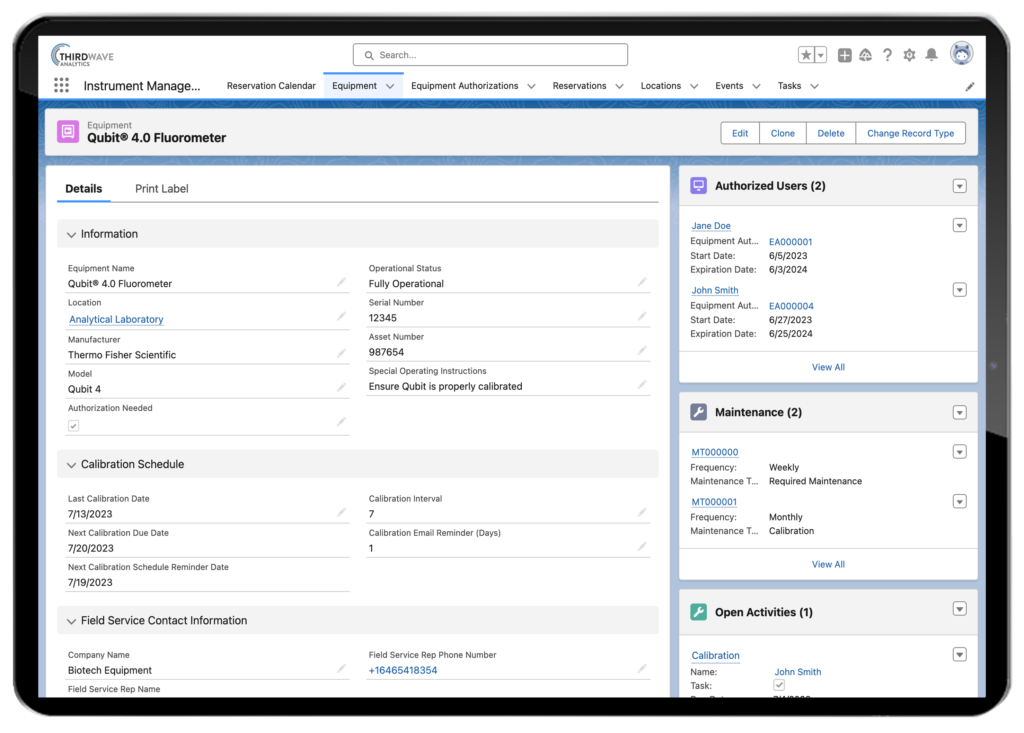
- Instrumentation Tracking. Every lab has a variety of instrumentation it uses daily, including liquid handlers, QC instrumentation, and sequencers. It is critical for a lab to track the maintenance and calibration these instruments use so the test data they generate is deemed accurate. A LIMS tracks all this activity and automatically alerts users when maintenance is pending or overdue. It also can track any issues that arise with lab instrumentation as they come up.
In addition to these critical features, LIMS software provides the reporting, analytics, and collaboration functionality to make all the data that is captured above useful. From a reporting standpoint, a LIMS can provide information on any aspect described above. Example reports include protocol duration, sample throughput, reagent expiration, and instrument uptime. Dashboards can be set up to provide high-level trending. For example, dashboards could display the number of submissions over time, lab staff utilization, and sample status.
A LIMS can also help an organization achieve compliance with 21 CFR Part 11, where required. It aids in managing automatic data collection, records, and electronic signatures. The LIMS may also offer functionalities that enforce data security, data integrity, audit trails, and other related procedures. Compliance with these regulations is crucial for laboratories operating within regulated industries such as pharmaceuticals, food and beverage, and environmental impact.
LIMS also assists in documentation management, another requirement for 21 CFR Part 11 compliance.
How a LIMS differs from an Electronic Laboratory Notebook
While they might seem similar initially, a LIMS and an Electronic Laboratory Notebook (ELN) differ and serve distinct roles within laboratory informatics.
While a LIMS streamlines laboratory operations by handling the management and logistics of samples, workflows, and data (structured, repeated workflows, processes, and data) – an electronic lab notebook is an electronic device that substitutes paper laboratory notebooks.
ELNs focus more on enhancing data management, research collaboration, and recording data. They are often associated with changing, configurable workflows and recording data.
While LIMS and ELN serve vital roles in managing laboratory data and operations, they are used differently and often complement each other. In some cases, an ELN might even be a component of a LIMS platform.
When to consider LIMS implementation
A LIMS has significant advantages for the modern laboratory, and many labs are now implementing solutions to update their lab technology.
Today’s labs process and analyze an incredible amount of information. Without a reliable and comprehensive LIMS system in place, it’s easy for errors to impact the speed and accuracy with which results are delivered. In some cases, this could mean a new product launch gets delayed — or that a life-saving vaccine urgently needed takes longer to develop. An online lab management system is crucial for organization, accuracy, and collaboration. So, suppose your lab uses outdated software, systems that don’t work together, Excel files, or even pen and paper to record data. In that case, it’s time to upgrade and implement a LIMS that works best for your industry, goals, and environment.
From a collaboration standpoint, a Laboratory Information Management System can also help a lab group stay connected. Tasks can be automatically assigned to various team members as a sample moves through a protocol. Documents can be annotated, shared, and commented on within the LIMS. Emails and phone calls can even be captured so communication amongst team members and external collaborators can be shared with everyone.
What is the best LIMS system on the market?
Well, that depends on the lab and its individual needs.
That said, with greater efficiency and organizational benefits, it’s clear why today’s most successful laboratories rely on a laboratory information management system like the highly-rated Lockbox LIMS. LIMS systems allow labs to organize sample tracking, workflow support, data management, and collaboration opportunities in a single piece of software, making it the lab’s backbone.
Learn more about Third Wave Analytics and our secure Lockbox LIMS platform; you can review the capabilities or try a Lockbox LIMS demo to explore the full features.