The Role of Quality Change Control in Laboratory Information Management Systems
In almost every industry, whether it involves running lab tests, manufacturing medical devices, producing food products, or developing drugs, it is mission-critical to keep track of all changes. In the life sciences industries, such as biotech, medical device, pharmaceutical, testing laboratories, or CLIA laboratories, the change control process is key to compliance efforts. Understanding why a change control system is so important highlights how an effective Laboratory Information Management System (LIMS) is essential for an orderly lab.
Why is a LIMS Important? Change Control and Your Lab
Change control is at the top of most auditor’s lists, as it is the basis of many regulations. The documentation and retention of data regarding change is a key audit “inspectable.” Failure to track and document changes, to sustain file integrity monitoring, and to retain those records for the appropriate amount of time, can result in inspection failures.
In change management, it is also necessary to track changes to ensure the consistency of your products and processes, as well as ensure that your customers are always receiving top-notch products and services. Change control happens at many levels in a regulated organization, including, but not limited to, document control, software change control, process control, calibration, inventory control and materials management, and protocol management. Changes may be documented in many ways, most commonly via an audit trail. This is why a flexible, user-friendly LIMS is the most important management solution you can implement.
Using a Change Control Software for Change Management
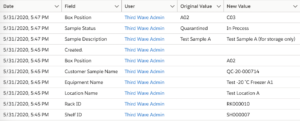
Third Wave Analytics Lockbox LIMS system comes “out-of-the-box” with audit trail and change management tools. By default, every screen (or object) can be set to show system information such as the owner, date, and time of the last modification. If more extensive audit trail functionality is required, this change control software can be configured to capture change log history for up to 60 data fields and to store that data indefinitely (with an add-on called Field Audit Trail). This information can also be encrypted to meet your HIPAA and GDPR requirements. Lockbox even has built-in reports that can help you present change metrics and audit trail data to inspectors.
In addition to all the great audit trail and change management capabilities built into Lockbox LIMS, your Quality Assurance team can rest easy knowing that Lockbox itself is built by a company, Third Wave Analytics, which employs the same quality and change management systems that you do. We take quality seriously. Our Quality Management System was created to ensure that we create products that are ready for validation in FDA, CLIA, HIPAA, GDPR, and ISO/IEC environments. Lockbox change control software is design-controlled, change-controlled, and version-controlled; and when implementing change to the system, all changes are risk-assessed, documented, and tested before they are ever released to customers. We even provide computer systems validation consulting services to help you remain in compliance during Lockbox upgrades, or when you want to add new functionality to your Lockbox LIMS.
For more information on how Lockbox LIMS (Laboratory Information Management System) and Third Wave Analytics can help you meet your control management requirements with our intuitive control management software, please contact us today.