Understanding the Key Benefits of Laboratory Information Management Systems
A Laboratory Information Management System or LIMS (also referred to as a Laboratory Information System) offers a variety of functions to manage your lab more efficiently. Each lab stakeholder use a LIMS for a different purpose, and will benefit from the LIMS in different ways.
Below, we review different LIMS functionalities and the value they bring to each of five groups at your lab.
LIMS Uses and Benefits for Laboratory Technicians and Sample Testing Personnel
Lab technicians and other sample testing personnel, such as sample accession personnel, are likely to be the most frequent and extensive LIMS users at your lab. They push your samples through your lab’s testing procedures, generate the data, and must document their actions within the LIMS. Therefore, this group is one of the most important stakeholders to consider.
Here are the key LIMS features that can benefit lab technicians:
Sample Management
For lab technicians to do their job effectively, they need to know the location of samples, the labels for any sample tubes/containers, and the testing status for each sample. Sample management that enables this work is, by far, one of the most important functions of a LIMS. Your lab techs will save an enormous amount of time if they can easily determine the samples that need testing, their locations, and their labeling.
Lockbox LIMS Sample Management
Sample Processing and Protocol Execution
Processing samples frequently presents a complicated workflow in many labs. This is due to either a difficult protocol or a single lab tech attempting to process a large number of samples at one time. A LIMS can deliver a tremendous benefit by providing accurate instructions for each step of the sample testing process. The LIMS can also easily record the protocol(s) performed on each sample, providing your staff an up-to-date list of where samples are in the testing process.
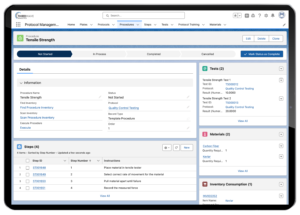
Lockbox LIMS Protocol Management
Data Capture and Analysis
Some protocols generate various data throughout testing (e.g. sample concentrations) or at final testing results (e.g. qPCR or ELISA data). Your lab may also need to perform analyses on many data files, so that samples can be tested. For instance, you may need to transform a sample concentration into the volume required for the sample to be tested, or transform a qPCR Ct value into a “Positive” or “Negative” call. Regardless of the situation, lab technicians must accurately “process” all the data generated from testing so that final results for all samples are accurate.
However, it’s easy to mix up, lose, or modify data files accidentally. This can be prevented with a LIMS, an excellent solution to minimize the pain these problems present, and prevent errors. Your lab technicians can upload raw and unaltered data files directly into the LIMS, which analyzes the data automatically. This can save a considerable amount of time and prevent a large number of errors.
Training
Lab techs must undergo an extensive amount of training to test samples. A lab must usually document this training in a specific manner. A LIMS is an ideal outlet to both perform and document the training. Your lab can store all testing documents that must be reviewed in the LIMS; you can also use the LIMS to easily maintain the training records for each individual lab tech.
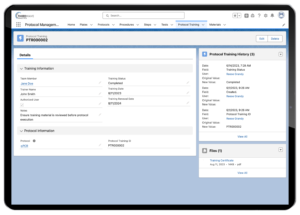
Lockbox LIMS Protocol Training Record
LIMS Uses and Benefits for Lab Managers and Lab Supervisors
Lab managers and supervisors are also likely to use a LIMS extensively at your lab. They keep track of how their lab is testing all samples, and ensure it’s being done in an accurate and timely manner. Some labs may have a complicated testing procedure or may test a large number of samples—playing traffic cop for all samples is a difficult task. Because they need to know where samples are in the process at all times, lab managers and supervisors will find a LIMS to be their best friend.
Here are some key LIMS features that greatly benefit lab managers:
Sample Management
Similar to lab techs, lab managers also have to manage samples within the LIMS; however, lab managers must do so at a higher level. Instead of knowing samples’ physical location, lab managers must know which step each sample is in within the testing process. How many samples or batches require testing at each step? What is the current sample turnaround time (TAT)? How many samples did the lab test last week compared to this week? A LIMS offers an effective way to keep track of and answer these important high-level questions.
Lockbox LIMS Sample Management Dashboard
Inventory Management
Lab managers often have to ensure their lab has enough resources on hand to test all samples. There is nothing worse for a lab than having to stop testing because you’ve run out of a critical reagent. With a LIMS, your lab managers can track the number of reagents currently in stock, and estimate how many you’re using for sample testing. The LIMS also gives you the ability to order reagents automatically, so your lab doesn’t have to remember to order each specific reagent.
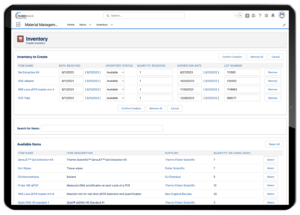
Lockbox LIMS Inventory Management
Scheduling
Sample testing is a complicated traffic jam. The process must be well-coordinated to ensure the required equipment and personnel are available to perform the required testing steps. A variety of equipment can be in high demand, or not always operational, and other personnel (such as researchers) may need to use your equipment. A LIMS improves the situation, allowing the lab manager to schedule your lab’s equipment and personnel across various stakeholders with far greater organization and ease.
Training
Lab managers are usually responsible for making sure lab techs have performed all required training. It’s an enormous and difficult job. In fact, for regulated labs, training documentation is usually the number one deficiency during an audit of the lab’s documents. A well-designed LIMS is one of the most powerful tools lab managers can use to know which trainings are required for which personnel, and which have been performed and documented.
LIMS Uses and Benefits for Lab Directors and Clinical Lab Scientists (CLS)
Lab directors and CLS’s play an important role, ensuring the lab tests all samples in an appropriate manner, and that all generated data is of sufficient quality. The lab techs generate the results and data, and the lab directors review it all. While lab directors might not spend as much time in a LIMS as a lab tech or manager, a LIMS does provide considerable benefits to the role:
Sample Review and Result Sign-out
Usually, the lab director’s most important job is reviewing sample testing data, and the sign-out and reporting of samples. However, data review can be a tedious and extensive process, and lab directors can be required to review a large number of samples every day. A well-designed LIMS can help a lab director simplify, manage, and expedite the entire review process.
Document Review and Approval
Many labs have dozens or even hundreds of “controlled” documents a lab director must review and approve. With a potential for an unwieldy number of document drafts and previous versions, performing and documenting appropriately is challenging. Furthermore, document approval is usually a stringent process that must be documented with formal signatures.
A LIMS can easily help lab directors sort through any possible confusion. It provides a clear path to identify which documents are ready to be reviewed for editing or approval, and which require no action from the lab director.
Quality Control (QC) Review
A lab must QC many reagents and instruments prior to use in sample testing, and a lab director must review the data. Additionally, a lab must constantly ensure that instruments are appropriate for testing (e.g. staff have cleaned instruments and performed preventive maintenance). A LIMS can both store this information and present it to the lab director for review. With the lab director responsible for reviewing an enormous amount of lab data, a LIMS provides an ideal way to present QC data for easy review and signoff.
LIMS Uses and Benefits for Quality Assurance (QA) and Quality Management Services (QMS)
Many labs have a significant regulatory burden to maintain a high level of quality throughout sample testing, and thoroughly document that quality. Personnel in QA and QMS have the challenge of ensuring that all samples have been tested appropriately. The mantra “It did not happen if it was not documented” is particularly relevant to QA and QMS, who must assure testing quality is documented in a way that is easy to review and audit.
Here are some key LIMS features to benefit QA and QMS personnel:
Sample Testing and QC Data
QA and QMS are generally responsible for ensuring the appropriate review and storage of all sample testing data and reagent/instrument QC data. This can require huge amounts of paperwork and data, which can be difficult to audit at a later date. The job becomes excessively challenging when a lab conducts documentation via paper records.
Therefore, a searchable LIMS is critical—the most painless way to ensure QA staff can meet this tedious requirement. A LIMS can alert your lab when paperwork is missing, and can be easily searched for auditing purposes.
Document Review and Approval
QA and QMS usually must confirm that all “controlled” documents are up-to-date, accurate, and approved. As stated above, a single lab can have dozens or hundreds of such documents. It is already challenging to know which documents have been approved, require approval, or need a scheduled re-review—along with which version of a document is active. If done with paper documents and via email, this responsibility is nearly impossible.
A LIMS may be the only real solution to manage your controlled documents appropriately. Personnel can easily search and filter information within a LIMS to know the status and active version of each document. A LIMS can also automatically alert you when documents require a scheduled re-review, and store all approvals for each document.
Lab Record Auditing
As part of their responsibilities, QA and QMS may need to audit all lab records including sample testing data, training records, and document access logs. For this need, a well-designed LIMS can be a dream when it’s time to perform an audit. QA and QMS can easily find all the records you need without having to search through piles of paper documents.
Additionally, a LIMS can also easily record whenever your lab updates data or records. This provides you an audit trail that details who updated a data record and when, and the new/old values.
LIMS Uses and Benefits for Research Personnel
Research personnel commonly perform extensive sample testing, albeit for research samples that are not reported. Nonetheless, lab research staff often benefit from much of the same LIMS infrastructure that help lab technicians and managers.
Here are some key LIMS features that can benefit research personnel:
Sample Management
Research personnel face a similar problem to lab technicians when they perform sample testing. As part of identifying all samples that currently require testing, the staff must find the sample locations and how they are labeled. Furthermore, they must be able to differentiate between research samples and clinical samples—a mix-up between the two could be problematic.
A LIMS prevents such errors and improves the process. It can easily perform concurrent sample management for both clinical and research samples without mixing the two.
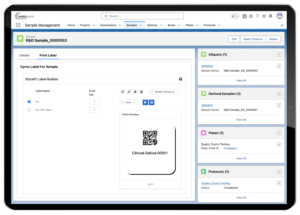
Lockbox LIMS Barcodes for Sample Management
Study Management
Research personnel commonly perform sample testing as part of research or a validation study. If they conduct multiple studies at the same time, sample tracking and data management can pose a problem. With a LIMS, research staff can see which samples and data are associated with each study.
Data Capture and Analysis
Capturing data and performing its subsequent analysis can be tricky and error prone; the problem is only amplified if your lab is assessing multiple data types or using multiple analysis methodologies. Research personnel can utilize a LIMS to store and easily query data without having to find all the files in some random folder on your company’s research drive.
Summary: Every Person In Your Lab Benefits from a LIMS in Different Ways
When evaluating a LIMS to implement at your lab, it is important to identify the features each user might require for their responsibilities. Using the recommendations above, review the LIMS functions most important for each of the following roles at your lab:
- Lab technicians and other sample testing personnel
- Lab managers and supervisors
- Lab directors and clinical laboratory scientists (CLS)
- Quality Assurance (QA) and Quality Management Services (QMS)
- Research personnel
Want to learn more? We’ll answer all your questions, from “What is a LIMS?” to “How can a LIMS improve my lab processes?” For answers and a free trial, contact our experts today.