Optimize Laboratory Quality Management with a Modern QMS
Summary
Data integrity, process standardization, efficiency, precision, and detailed record-keeping are essential for the success of any laboratory. These attributes are earnestly pursued by laboratories dedicated to quality. In the process, lab managers are often challenged by inconsistent procedures, regulatory compliance hurdles, inefficient paper-based systems, and difficulties in maintaining accurate training records. These issues can lead to errors, non-compliance, and operational inefficiencies. A Quality Management System (QMS) is designed to address these common problems, ensuring that quality is consistently maintained. Our Lockbox QMS allows your lab to improve efficiency, meet compliance regulations, and build trust in your laboratory operations by providing a robust solution to these issues.
Key Take Aways
- A QMS is a formalized system that documents and tracks the processes, procedures, and people responsible for maintaining quality standards
- With Lockbox QMS, labs can transition away from traditional paper or spreadsheet-based quality management to a modern digital system
- A QMS system helps laboratories enhance compliance with regulatory guidelines including ISO 17025, CAP/CLIA, and FDA 21 CFR Part 11
- A QMS simplifies audits by storing and organizing all controlled documents, quality event records, and training records in one secure location
- Lockbox QMS integrates seamlessly with Lockbox LIMS, allowing for comprehensive lab and quality management in one system
- Lockbox QMS can be used with Lockbox LIMS or as a standalone product
What Does a Laboratory QMS Do?
A laboratory quality management system (QMS) is a formalized system that documents and tracks the processes, procedures, and people responsible for maintaining quality standards in a lab. A QMS is designed to ensure an organization’s products or services meet customer and regulatory requirements and continue to improve their efficiency and effectiveness. A lab QMS manages and standardizes processes to ensure consistent quality outcomes.
It helps labs achieve and maintain regulatory compliance and meet industry standards such as ISO 17025 and 13485, CAP/CLIA, GMP, GLP, HIPAA, and 21 CFR Part 11/EU Annex 11.
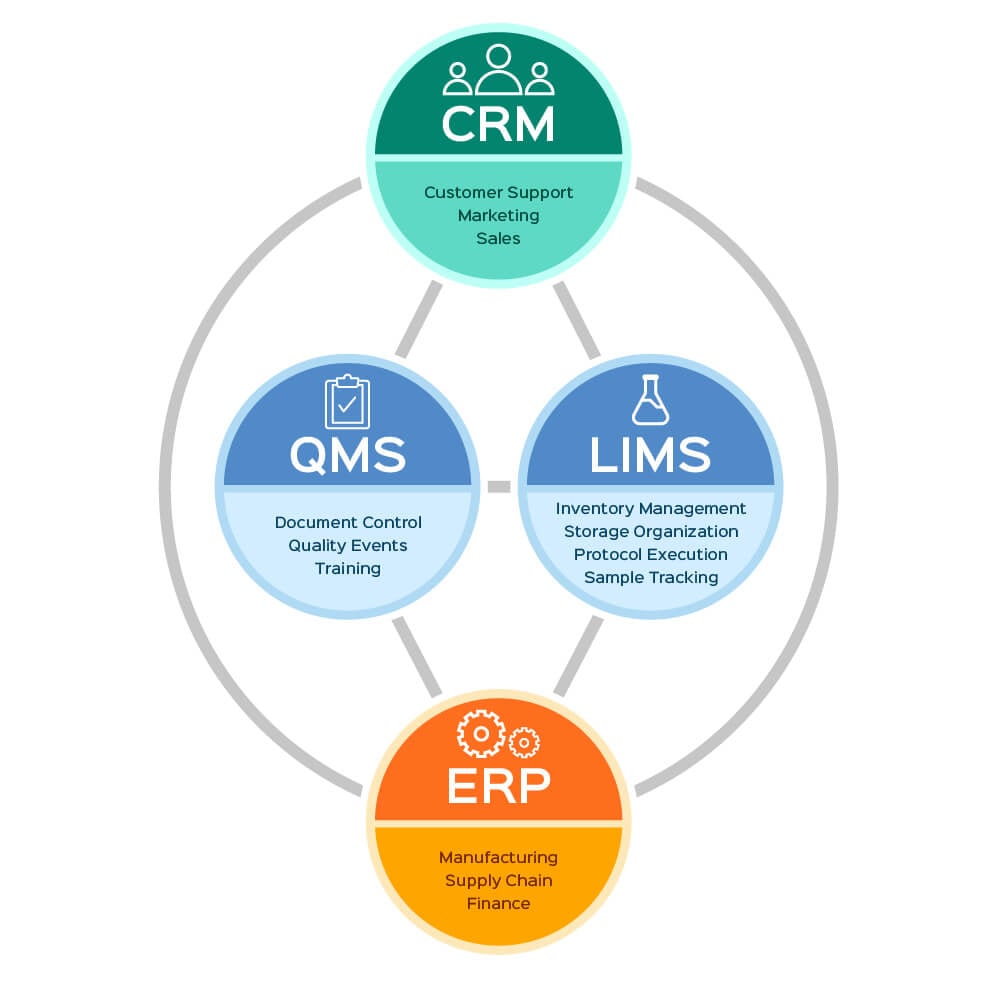
Most Laboratory QMSs, including Lockbox QMS, provide the capacity to manage controlled documents and versions, document quality event incidents and responses, and track training requirements and compliance. Lockbox QMS includes Controlled Documents, Quality Events, and Training as individual modules that can be utilized together or separately. The linked articles describe these modules in detail.
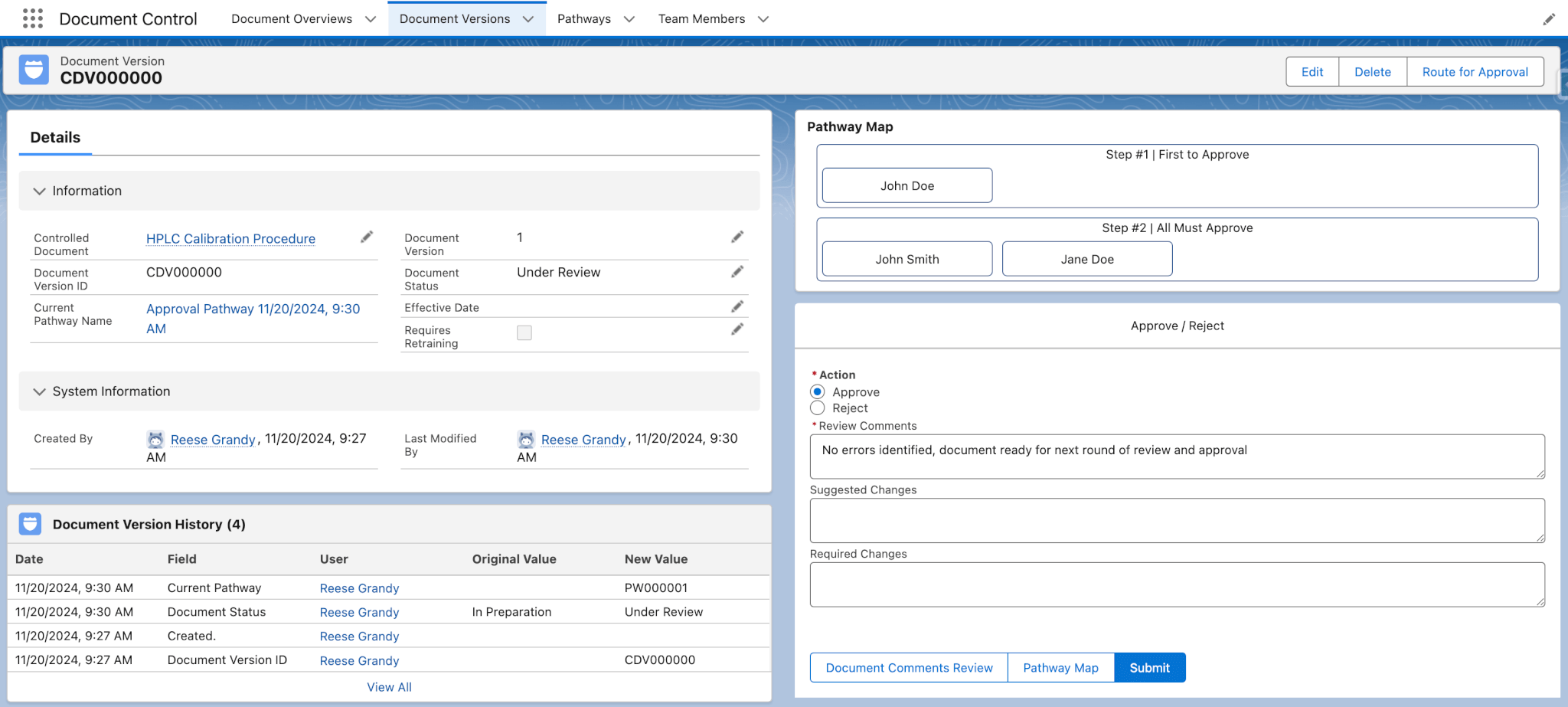
Document Control
This module allows users to track controlled documents and their versions, and route them for approval. It doesn’t replace your favorite document editing tool but tracks all changes to the controlled documents that are important to your organization. With Lockbox QMS Document Control, users can easily and quickly find the most current document version, reducing the risk of errors.
Quality Events
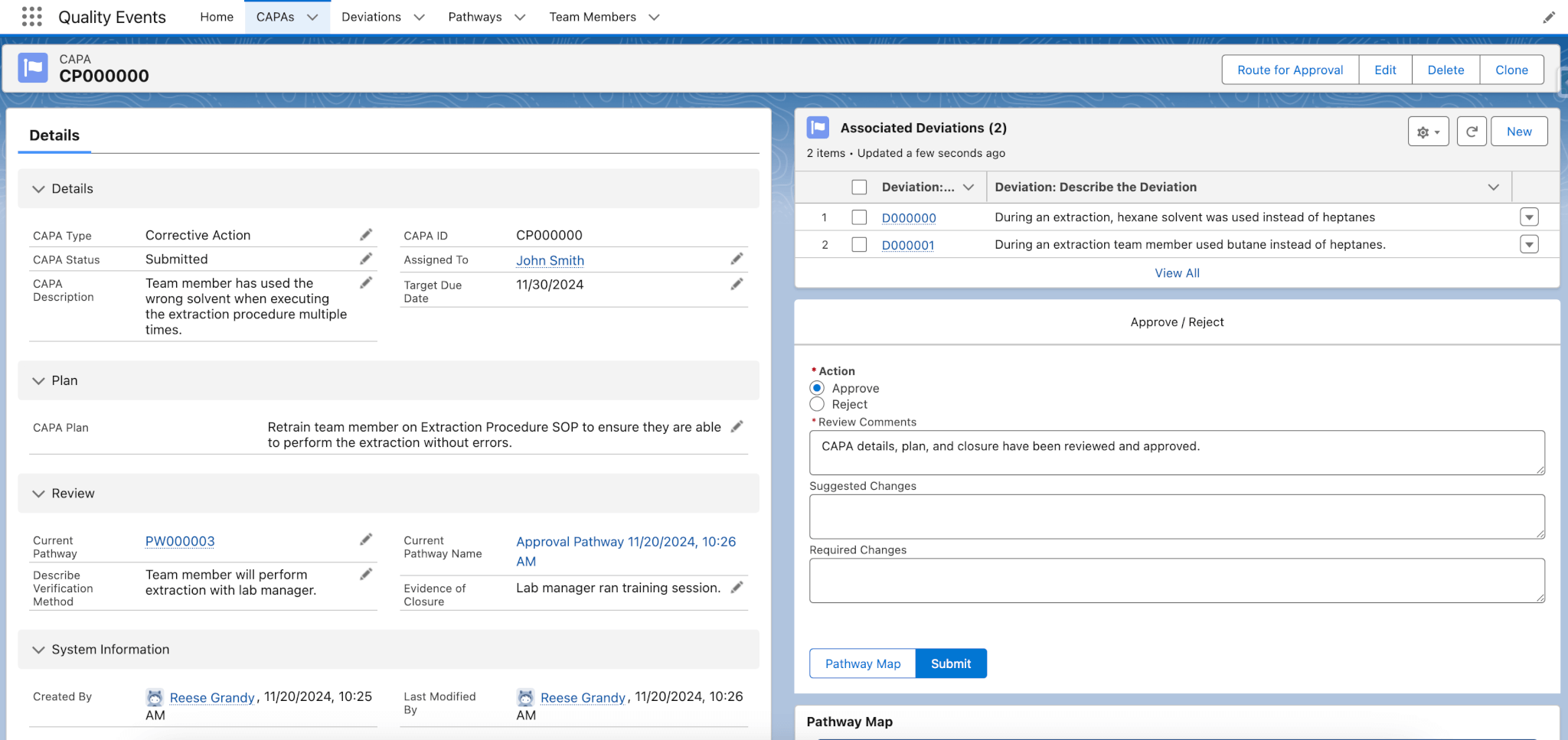
The Quality Events module tracks deviations, CAPAs (Corrective And Preventive Action), and risks. Users can log quality-related events in real-time, allowing managers to promptly address deviations and CAPAs, enhancing your lab’s compliance and operational efficiency. Lockbox QMS Quality Events guides users through the process of documenting the event, assigning it to relevant personnel, and tracking resolution steps.
Training
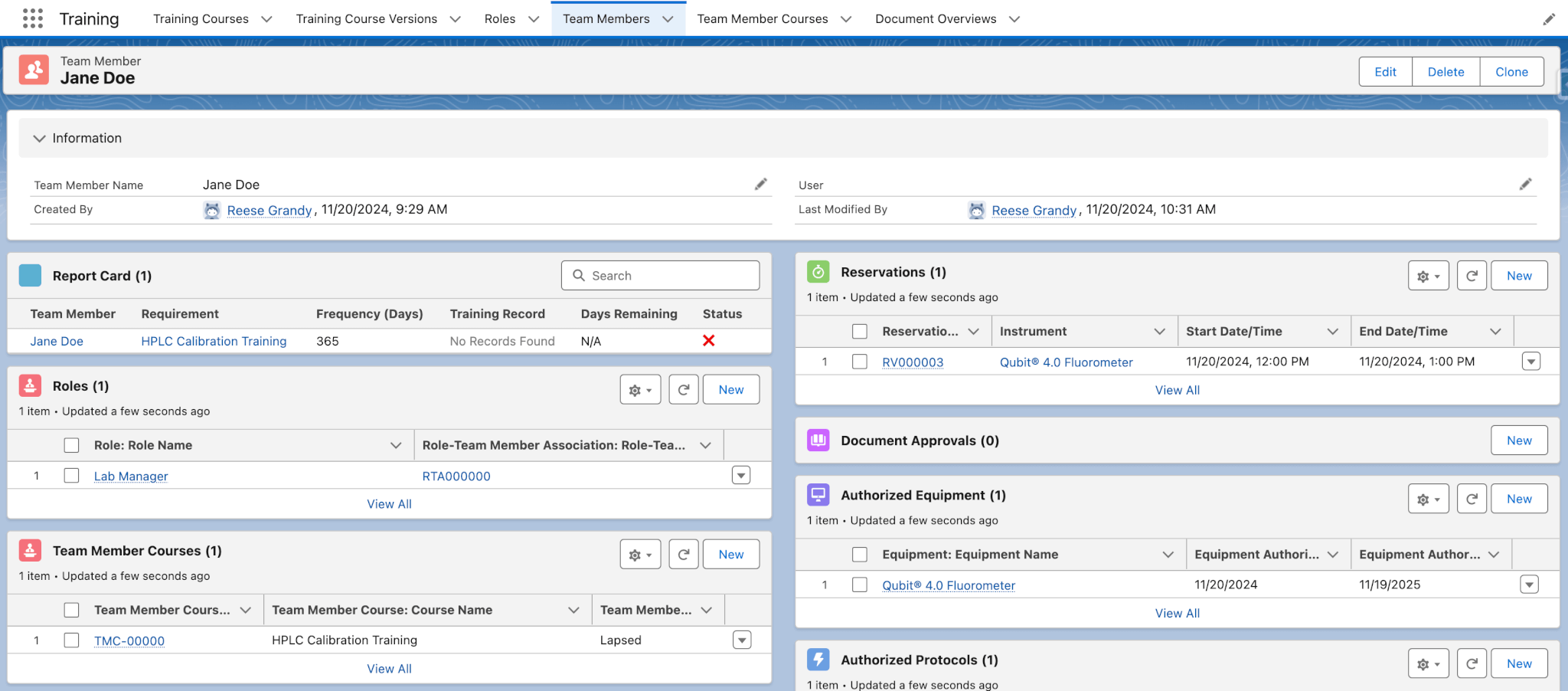
The Training module simplifies the management of your team’s training requirements and history, which can be a daunting task in a busy laboratory. Lockbox QMS Training tracks training data, including team members, roles and associated curriculums, training courses, approved trainers, training course versions, and training records. This module is not a mechanism for delivering training to team members, but for tracking training progress. If a new protocol or instrument comes to your lab for a project with a tight turnaround, managers can quickly and easily assign new training requirements with Lockbox QMS Training.
How a QMS Enhances Laboratory Operations
Implementing a QMS in a laboratory setting provides many operational benefits that enhance efficiency, compliance, and reliability. Here’s how Lockbox QMS can improve your laboratory operations.
Audit Preparedness
A QMS ensures that all necessary documentation, including controlled documents, quality event records, and training records, are well-organized and easily accessible. A digital QMS allows users to maintain thorough and accurate records, simplifying the audit process, and making it easier for laboratories to demonstrate compliance with regulatory standards and pass audits with minimal disruption.
Quality event tracking, document control, training management, and change control are requirements in many compliance guidelines, including ISO17025, ISO15013485, ISO15189, US FDA 21 CFR Part 11, and CAP/CLIA. Lockbox QMS helps laboratories achieve these and other regulatory guidelines by providing robust tracking and reporting capabilities, customizable features, data retention, and audit trails. Lockbox QMS can simplify quarterly review meetings and keep materials needed for audits in one central digital location.
Workplace Safety
Lockbox QMS includes training modules that ensure all personnel are adequately trained on safety protocols and procedures, fostering a safe work environment and a safety culture. Using a QMS simplifies the tracking of training records and automates training requirement reminders.
Process Standardization
Lockbox QMS assists quality assurance professionals in ensuring that all lab procedures are standardized and followed accurately and consistently. This minimizes variability and errors in testing and research. Lockbox QMS gives laboratories an organized digital solution so they can provide their employees with clear and documented standard operating procedures and protocols that all personnel can follow.
Risk Management
A QMS enables users to proactively identify potential risks that could impact quality, safety, or compliance, enabling the laboratory to mitigate these risks before they become issues. Lockbox QMS facilitates the documentation and implementation of quality events, including CAPAs and deviations. The tracking of quality events allows users to promptly address any deviations or non-conformities, helping to prevent recurrence and improve overall quality.
Continuous Improvement
A QMS fosters a culture of continuous improvement within your organization by encouraging regular evaluation of processes and outcomes, thus facilitating ongoing enhancements in laboratory practices. Utilizing Lockbox QMS leads to time-saving tracking and reporting capabilities, allowing lab managers and directors to always have a quality snapshot available when needed.
The Role of Technology in Supporting a Laboratory QMS
Many laboratories are still utilizing paper or spreadsheet-based QMS. Embracing technology in the modern laboratory is critical to creating a seamless flow of data and enhancing overall laboratory management. Using paper or spreadsheets-based quality management systems is not only time-consuming it can lead to the loss of key data or information. The digital transformation within a laboratory is necessary for future-proofing laboratory operations and ensuring scalability as your laboratory grows. Utilizing Lockbox provides a digital QMS and LIMS solution that allows laboratory staff to focus on their core responsibilities, enhancing productivity and operational efficiency.
Future-Proofing and Scalability
Embracing digital transformation keeps your lab at the forefront of quality management practices and ensures scalability as your lab grows.
Integration with LIMS
A modern LIMS can complement a QMS by providing a seamless flow of data and enhancing overall laboratory management. The pre-integration between Lockbox LIMS and Lockbox QMS can prevent lab technicians from running outdated protocols or using equipment without having the required certification.
Automation and Data Management
Leveraging technology enhances accuracy, efficiency, and reporting. Automation of laboratory processes improves accuracy, reduces manual errors, and ensures consistent quality. Additionally, a digital repository replaces the traditional paper-based binders-on-bookshelves method. Lockbox QMS is a powerful asset for quality management departments, enabling them to simplify quarterly quality meetings, maintain continuous audit readiness, and achieve compliance requirements.
Instantaneous Reporting
A digital QMS provides laboratories with many more features than a paper or spreadsheet-based quality management system. Users can generate reports on CAPAs and deviations, manage which employees have completed training, and track how long revised documents take to get approved. Users do not have to dig through spreadsheets or paper documents and create reports by hand. Lockbox QMS users can let Lockbox do the tracking so the users can focus on their jobs.
Why Implement Lockbox QMS?
Our customers asked and we delivered. Third Wave Analytics developed Lockbox QMS (Quality Management System) in response to our customers’ needs and feedback. Our customers wanted to streamline lab processes and consolidate their quality management processes into Lockbox to continue their digital lab transformation. Lockbox QMS seamlessly integrates with Lockbox LIMS, providing the ability to manage controlled documents, track and report quality events, and manage employee training, all in one secure, cloud-based system.
Lockbox QMS and Lockbox LIMS can be purchased and used together or separately, depending on the needs of your laboratory. Since Lockbox QMS and Lockbox LIMS are built on the same platform, they can be purchased and used as a unified, pre-integrated system. Lockbox LIMS Sample Tracking, Instrument/Equipment Management, Inventory, and Protocol Execution modules can all be connected to Lockbox QMS. Utilizing the enhanced tracking and reporting capabilities provided by this integration, users can fully track protocol or standard operating procedure versions and see the full impact of a deviation, CAPA, or past-due training. Using Lockbox LIMS and Lockbox QMS together allows users to quickly and easily identify the impact of bad reagents, protocol execution errors, and lab equipment malfunction. Users can quickly notify patients whose tests were affected, flag affected products, and queue affected samples for re-processing.
Like Lockbox LIMS, Lockbox QMS is built on the Salesforce platform, giving users the same security features, ease of use, and a high degree of customization that our customers know and love. No Salesforce or coding experience is required to use Lockbox QMS effectively. Lockbox QMS data integrity and traceability are achieved in the same way as in Lockbox LIMS, through audit trails and data retention. Like Lockbox LIMS, Lockbox QMS integrates seamlessly with business applications and onsite devices.
Third Wave Analytics has built Lockbox LIMS and Lockbox QMS as an all-in-one solution. These products incorporate unique features and customization allowing our systems to bend to fit your laboratory processes, not the other way around. Third Wave Analytics has a unique team committed to understanding and finding solutions for your specific laboratory needs.
Labs That Benefit from Implementing Lockbox QMS
Many clinical, testing, manufacturing, and regulated labs already have some type of QMS system in place. Lockbox QMS provides a modern, easy-to-use digital upgrade from common paper-based systems, and easily integrates with Lockbox LIMS and other systems.
Quality tracking, document control, training competency, and change control are requirements in many compliance guidelines including ISO17025, ISO15013485, ISO15189, US FDA 21 CFR Part 11, and CAP/CLIA. Lockbox QMS helps laboratories achieve these and other regulatory guidelines by providing robust tracking and reporting capabilities, customizable features, data retention, and audit trails. Lockbox QMS can simplify quarterly review meetings and keep materials needed for audits in one central digital location.
Lockbox QMS Training module aids laboratories in creating a culture of safety within their organization by simplifying training records and automating training requirement reminders. Lockbox QMS Training module gives lab managers and C-suite staff a 360-degree view of their personnel training compliance.
Conclusion
Lockbox QMS is a cost-effective, robust, and easy-to-use system that is pre-integrated with Lockbox LIMS, so all your lab management systems will be in one central location. Users never need to leave Lockbox. Lockbox QMS facilitates digitizing your lab processes so that your lab team can focus on what matters most – the science. Let Lockbox QMS assist your lab in building a reputation for reliability and excellence through consistent quality management. Gain a competitive edge by demonstrating a commitment to high standards and continuous improvement.
If you are ready to learn more, contact us and see how a laboratory quality management system can transform your laboratory operations.